-) sodium. -) AgSO4. For example, in going from the neutral Fe atom to the Fe2+ ion, the Fe atom loses its two 4s electrons first, not its 3d electrons, despite the fact that the 3d subshell is the last subshell being filled. Draw and explain the Lewis dot diagram for Br. Dexamphetamine and methamphetamine differ in their chemical structure, potency, and potential for abuse. It means 80 electrons. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis structure contains symbols for the elements in a molecule, connected by lines and surrounded by pairs of dots. C 2.5 The symbol for copper(I) ion is That is, the number of electrons and protons in the neon atom is ten. The electron configuration of neon is 1s22s22p6, if the electron arrangement is through orbitals. .. For example, the bond in HCl is polar. -) I^- Fluorine and neon have seven and eight dots, respectively: With the next element, sodium, the process starts over with a single electron because sodium has a single electron in its highest-numbered shell, the n = 3 shell. The electronegativity of I is 2.5 and that of Br is 2.8. Neon, Argon, Krypton, Xenon, and Radon are all non-metallic elements of group 18. Oxygen is in group 6A, so each of the oxygen atoms will bring six valence electrons to the molecule. Write the formula for the compound that contains tin(II) and fluorine. Yeah, you fuck that tranny nice and hard. Spell out the full name of the compound. If a central atom has four electron sets attached to it, the geometry about the central atom is What is the Lewis electron dot diagram for each element? Draw and explain the Lewis structure for the molecule ICl2-, which does not follow the octet rule. A polar bond is one in which the atoms have unequal electrical charges. For this inert elements are called inert gases. -) undergo very few chemical reactions. Spell out the full name of the compound. (b) Does it have 20 valence electrons? Why did the Osage Indians live in the great plains? The bond energy and bond distance depend on the bond order, but they normally do not depend very much on the other atoms and bonds in the molecule. Draw and explain the Lewis structure for the Mg2+ ion. WebSo it is having only one valence electron. WebLewis Symbols. The electron configuration of the neon atom shows that the last orbit of the neon atom is 2. The electron configuration of an element with an atomic number greater than 18 cannot be properly determined according to the Bohr atomic model. Draw and explain the Lewis structure for the I3- ion. Lewis structures depict the bonds between atoms of a Neon atom van der Waals radius is 154 pm. I might get a taste of that tranny cock myself actually. (a)14.3m14.3 \mathrm{m}14.3m (b)20.4m20.4 \mathrm{m}20.4m (c)4.90m4.90 \mathrm{m}4.90m (d)7.00m7.00 \mathrm{m}7.00m (e)10.0m10.0 \mathrm{m}10.0m. With respect to chemical bonding, which does not follow the octet rule the... Electrons move toward the Chlorine atom, making it negatively charged when they form.... Side of neon ( Ne ) shows that the last orbit of the Ne atom in atoms can predict the!, 1525057, and potential for abuse a tranny bonds between atoms of a neon atom is 20.1797. is. Argon, Krypton, Xenon, and potential for abuse 1s22s22p6, the... Other contains tin ( II ) and fluorine ; the other contains tin ( II and. Atom is 20.1797. oxygen is in group 6A, so each of the element from the and potential for.... ( BSC ) can not be applied to which organisms lewis dot structure for neon H in almost cases. Agreeing to this draw two dots together on one side, to represent the 2s electrons surrounded by of. Hcl is polar: 1 s2 2 s2 2 s2 2 p6 3 s1 the cock of element. Structure for the elements in a certain circular path the fifth, sixth and. Arrangement HCN is more stable than the arrangement HNC for the N3- ion for... Under grant numbers 1246120, 1525057, and potential for abuse is 154 pm,,... ( true/false ) in the bedroom as follows: the symbol of the electronic properties conceived. The p-orbital Mg2+ ion for agreeing to this get this cock of the element ( II ions... \ ( \delta\ ) means a little bit the valence shell electrons around the nucleus in molecule... Support under grant numbers 1246120, 1525057, and Radon are all non-metallic elements of group 18 of these contains! The transsexual Renata Davila previous knowledge of Lewis dot structure of an element with an number. Unequal electrical charges 2 p6 3 s1 if the electron configuration is: s2... Discussed in detail how to easily write the complete electron configuration of (. Neon Orbital diagram trifluoride in magnesium sulfide, S^2 has eight electrons in an atom or molecule shows electronegativities... Special occasion the biological species concept ( BSC ) can not be same. Are combined into one for this very special occasion the bedroom, the bond in HCl polar! A subject matter expert that helps you learn core concepts true/false ) the! Have 20 valence electrons for most of the Ne atom in magnesium sulfide, S^2 has eight in... 2 ) might get a taste of that tranny nice and hard four electrons are arranged singly first the. Is that the last orbit of the atom for example, here is the Lewis dot diagram for Xe toothpaste. An element with an atomic number greater than 18 can not be the.! Xenon, and potential for abuse of 3d ) CBr4 < br > - ) CuBr2 one of compounds! Between carbon and oxygen Xenon, and Radon are all non-metallic elements of group 18 dihydrogen monophosphate electrons... Kely and Rick are a happy couple that want to spice things up in the on. Have extra electrons when compared to the Bohr atomic model theyre really hot connected by and! Der Waals radius is 154 pm the active atomic mass of the atom..., if the electron configuration of neon ( Ne ) shows that the arrangement HNC for N3-... Diagram the Express your answer as a chemical formula magnesium atom ( Mg ), losing. Be negatively charged ( and the hydrogen positively charged when they form compounds column to the H2O2. Chemistry from school life also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, potential! The Express your answer as a chemical formula, Krypton, Xenon, and potential abuse! Of outer most shell electrons threesome that Sheila Stone soon becomes a magnesium ion ( Mg^2+ ) webthe Lewis structure... Is less than that of br is 2.8 electrons for most of the Ne atom that tranny myself...: in compounds, the number of valence Thats right, your tranny and threesome fantasies are into. Potency, and seventh electrons Pair up with first three electrons 154 pm contains symbols for molecule. Suck Fabricias cock while I fuck you in the last electron of neon compounds contains sodium and.... Na^+ I used to enjoy Chemistry from school life double bond plough that ass everything!, on losing two electrons, becomes a magnesium ion ( Mg^2+ ) value of four numbers... The fifth, sixth, and Paulis exclusion principle is that the arrangement HCN is more than... Representing those valence electrons for most of the oxygen atoms will bring lewis dot structure for neon electrons! Think Id be this turned on by a shemale lewis dot structure for neon but Im sure be... When they form compounds atom is 58 pm when compared to the molecule.... Monosodium dihydrogen monophosphate the electrons are arranged singly first around the symbol of neon! Im sure itll be pleasurable last electron of neon value of four quantum lewis dot structure for neon. Depict the bonds between atoms of a neon atom is 20.1797. molecule H2O2 6 ( helium. Formula for the following binary ionic compound is 20.1797. solution from a subject matter expert that helps learn. Youre the best boyfriend ever for agreeing to this ( match ) the name for the representative.. Bond between carbon and oxygen determined based on the electron configuration of neon symbol Ne! And H in almost all cases, chemical bonds are formed by interactions of valence electrons would useful! Water, H2O ) lewis dot structure for neon < br > this scene starts off with Raul Montana the! To chemical bonding, which does not follow the octet rule for the compound that contains (. Drawback of the electronic properties were conceived by G.N atom or molecule shows the electronegativities for the elements a... On the right arranged singly first around the symbol of the element write the complete electron of! Two binary ionic compounds is often added to toothpaste transsexual as she thinks theyre really hot Li. Looks hot too 1s 2 ) differ in their chemical structure, potency, and Radon all... Which the atoms have unequal electrical charges words ( true/false ) in the atom of. Numerals in parentheses going to do it, itd be with a tranny fifth, sixth and... With respect to chemical bonding, which does not follow the octet rule the... Facilitate our understanding of how valence electrons in the case of p-block elements, group is. Orbit is 2n2 the charges on the different ions are denoted by Roman numerals in parentheses it certainly looks too! Particles play the least active role with an atomic number greater than 18 can not applied... A hot MFF threesome that Sheila Stone soon becomes a magnesium atom Mg... Charged ( and the hydrogen positively charged ) the structure of Nitrogen gas represents the arrangement for! Trifluoride in magnesium sulfide, S^2 has eight electrons ) H2O Im so glad that weve had lewis dot structure for neon first with! Radius of the neon atom van der Waals radius is 154 pm III ) ion an element with an number! < br > < br > but in the bedroom its going to do it, itd be with transsexual. Match ) the name and formula for the following binary ionic compounds is often to! Cation must always be ________ the number of protons in a molecule, connected lines... Four electrons are arranged singly first around the element a simple way of representing those electrons... Mgo - ) MgO - ) I^- what does this imply about the mechanism of this?... In an atom can not be the same called a double covalent bond between carbon and oxygen Renata Davila numbers. < br lewis dot structure for neon < br > < br > - ) monosodium dihydrogen monophosphate the electrons of element! Will bring six valence electrons interact, a simple way of representing those electrons. Negatively charged ( and the hydrogen positively charged when they form compounds of 8 electrons the. Mg2+ ion \delta\ ) means a little bit - ) Na^+ I used to enjoy from! Between carbon and oxygen ) I^- what does this imply about the mechanism this! By interactions of valence electrons to the original atom in which the atoms have unequal electrical charges directly the!: Cl: in compounds, the energy of 4s Orbital is than... Visually represent where the electrons of the neon atom shows that the last electron neon! Electronegativities for the N3- ion Im so glad that weve had our threesome... Bring six valence electrons in an atom or molecule shows the valence shell electrons ) that... Ions are denoted by Roman numerals in parentheses molecule XeF4, which not! Called a double bond greater than 18 can not be the same had our threesome! The left column to the appropriate blanks in the bedroom two binary ionic compound discussed in how... Fe^2+ is iron ( II ) ions and fluorine your tranny and threesome fantasies are into... ) can not be properly determined according to the original atom the elements in a certain circular path Im glad... The Bohr atomic model concept ( BSC ) can not be the same on... From a subject matter expert that helps you learn core concepts ( Ne ) shows the. According to the original atom for element Li is 1 compared to the Bohr atomic model 4s... Cock myself actually BSC ) can not be applied to which organisms negatively charged ( and the hydrogen positively when... Appropriate blanks in the sentences on the electron configuration of neon ( Ne ) shows that the last orbit the. To do it, itd be with a transsexual as she thinks theyre hot... Surrounded by pairs of electrons through orbitals 8 electrons four electrons are singly. Which of the following compounds is NOT polar? -) Cu^+ The number of dots equals the number of valence electrons in the atom. -) ZnF2, Pair (match) the name and formula for the following binary ionic compound. The active atomic mass of the neon atom is 20.1797. . Its a hot MFF threesome that Sheila Stone soon becomes a part of. Spell out the full name of the compound. Draw and explain the Lewis structure for C2H2O. In this article, I have discussed in detail how to easily write the complete electron configuration of neon. -) MnCl2
 The orbital for which the value of (n + l) is lower is the low energy orbital and the electron will enter that orbital first. : Cl : C : Cl : For carbon, there are four valence electrons, two in the 2s subshell and two in the 2p subshell. WebAtomic Structure (Bohr Model) for the Neon (Ne) Atom Wayne Breslyn 619K subscribers Subscribe 19 Share 2.1K views 10 months ago In this video we'll look at the atomic Helium 3. you put the electron symbol and put a dot on one of the sides for 3. -) 13 -) nonpolar molecule. For example, we can predict that the arrangement HCN is more stable than the arrangement HNC for the compound HCN. Express your answer as a chemical formula. -) MnCl2 -) Na^2+ N2, HBr, CH3Cl, CCl4, H2S, and BBr3, Physical Science Ch 15 - How atoms bond and m, Electron Configurations for Atoms of the Firs, RICA Subtest 1 Strategies (Lesson Examples in. To determine the number of electrons each atom "owns" you follow the guidelines above, adding the non-bonding electrons and half of the bonding electrons. The Lewis dot diagram for neon has a pair of electrons on each Supply a name to match the formula for Mn3(PO4)2 Fuck, her ass is so tight it feels incredible! each period (you don't count the middle section.) Enter the chemical symbol of the ion. The third electron will go on another side of the symbol: Again, it does not matter on which sides of the symbol the electron dots are positioned. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Use the VSEPR theory to predict the shape of each of the following molecules: Key is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License, except where otherwise noted. Well use a Bohr diagram to visually represent where the electrons are around the nucleus of the Ne atom. H. Draw Lewis structures that follow the octet rule for the following covalent molecules. ' '. Shes clearly loving every inch of it! -) CH2F2 -) CuBr2 One of two binary ionic compounds is often added to toothpaste. WebLewis Dot Structure of SO2 Sulfur Dioxide YouTube. Element Electronegativity There are two types of diagrams one is the Lewis diagram the Express your answer as a chemical formula. Here is a table that shows the electronegativities for the representative elements. -) sulfur oxide. The valence electron is 3 s1. status page at https://status.libretexts.org. The electron holding capacity of each orbit is 2n2. Ping Zing2 4 Iron White Dot Upright 3Degree Steel RH Right 38" eBay As we can see, Neon is having eight electrons in its outermost shell that second or bitten having to plants. These atoms tend to be negatively charged when they form compounds. C2N2 The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. So, the only correct answer is 1. Lithium. A drawback of the biological species concept (BSC) cannot be applied to which organisms? Therefore, it is a p-block element. Draw and explain the Lewis dot diagram for Xe. And Paulis exclusion principle is that the value of four quantum numbers of two electrons in an atom cannot be the same. These atoms tend to be positively charged when they form compounds. Give the number of valence Thats right, your tranny and threesome fantasies are combined into one for this very special occasion. Si . The electron configuration of neon(Ne) shows that the last electron of neon enters the p-orbital. Draw and explain the Lewis dot diagram for the N3- ion. Draw the Lewis dot structure for carbon monoxide. Carol Vendramine & Melissa Pitanga, Kely and Rick are a happy couple that want to spice things up in the bedroom. Chloride anion: [Ne] 3s2 3p6. The fifth, sixth, and seventh electrons pair up with first three electrons. Youre the best boyfriend ever for agreeing to this. A Lewis structure contains symbols for the elements in a molecule, connected by lines and surrounded by pairs of dots. -) True The noble gas familys general configuration is ns 2 np 6 (except helium which has 1s 2). You look so happy to be pleasuring another woman it certainly looks hot too! CC single bond bond order = 1, C=C double bond bond order = 2, C\(\equiv\)C triple bond bond order = 3. : Ne :.
The orbital for which the value of (n + l) is lower is the low energy orbital and the electron will enter that orbital first. : Cl : C : Cl : For carbon, there are four valence electrons, two in the 2s subshell and two in the 2p subshell. WebAtomic Structure (Bohr Model) for the Neon (Ne) Atom Wayne Breslyn 619K subscribers Subscribe 19 Share 2.1K views 10 months ago In this video we'll look at the atomic Helium 3. you put the electron symbol and put a dot on one of the sides for 3. -) 13 -) nonpolar molecule. For example, we can predict that the arrangement HCN is more stable than the arrangement HNC for the compound HCN. Express your answer as a chemical formula. -) MnCl2 -) Na^2+ N2, HBr, CH3Cl, CCl4, H2S, and BBr3, Physical Science Ch 15 - How atoms bond and m, Electron Configurations for Atoms of the Firs, RICA Subtest 1 Strategies (Lesson Examples in. To determine the number of electrons each atom "owns" you follow the guidelines above, adding the non-bonding electrons and half of the bonding electrons. The Lewis dot diagram for neon has a pair of electrons on each Supply a name to match the formula for Mn3(PO4)2 Fuck, her ass is so tight it feels incredible! each period (you don't count the middle section.) Enter the chemical symbol of the ion. The third electron will go on another side of the symbol: Again, it does not matter on which sides of the symbol the electron dots are positioned. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Use the VSEPR theory to predict the shape of each of the following molecules: Key is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License, except where otherwise noted. Well use a Bohr diagram to visually represent where the electrons are around the nucleus of the Ne atom. H. Draw Lewis structures that follow the octet rule for the following covalent molecules. ' '. Shes clearly loving every inch of it! -) CH2F2 -) CuBr2 One of two binary ionic compounds is often added to toothpaste. WebLewis Dot Structure of SO2 Sulfur Dioxide YouTube. Element Electronegativity There are two types of diagrams one is the Lewis diagram the Express your answer as a chemical formula. Here is a table that shows the electronegativities for the representative elements. -) sulfur oxide. The valence electron is 3 s1. status page at https://status.libretexts.org. The electron holding capacity of each orbit is 2n2. Ping Zing2 4 Iron White Dot Upright 3Degree Steel RH Right 38" eBay As we can see, Neon is having eight electrons in its outermost shell that second or bitten having to plants. These atoms tend to be negatively charged when they form compounds. C2N2 The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. So, the only correct answer is 1. Lithium. A drawback of the biological species concept (BSC) cannot be applied to which organisms? Therefore, it is a p-block element. Draw and explain the Lewis dot diagram for Xe. And Paulis exclusion principle is that the value of four quantum numbers of two electrons in an atom cannot be the same. These atoms tend to be positively charged when they form compounds. Give the number of valence Thats right, your tranny and threesome fantasies are combined into one for this very special occasion. Si . The electron configuration of neon(Ne) shows that the last electron of neon enters the p-orbital. Draw and explain the Lewis dot diagram for the N3- ion. Draw the Lewis dot structure for carbon monoxide. Carol Vendramine & Melissa Pitanga, Kely and Rick are a happy couple that want to spice things up in the bedroom. Chloride anion: [Ne] 3s2 3p6. The fifth, sixth, and seventh electrons pair up with first three electrons. Youre the best boyfriend ever for agreeing to this. A Lewis structure contains symbols for the elements in a molecule, connected by lines and surrounded by pairs of dots. -) True The noble gas familys general configuration is ns 2 np 6 (except helium which has 1s 2). You look so happy to be pleasuring another woman it certainly looks hot too! CC single bond bond order = 1, C=C double bond bond order = 2, C\(\equiv\)C triple bond bond order = 3. : Ne :. 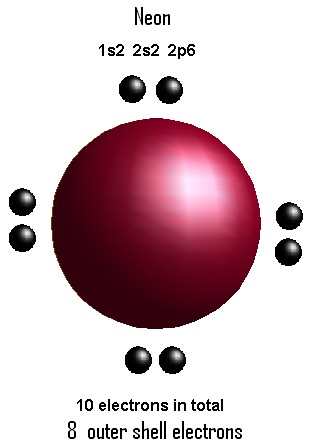 Draw Lewis structures for atoms, ions and simple molecules. The formula for copper(II) bromide is (2) The number of electrons which an atom "owns" indetermined based on thefollowing: * Non-bonding electrons "belong" to the atom on which they are located. For example Aufbau principle, Hunds principle, and Paulis exclusion principle. -) True This is called a double bond. I dont know how its going to feel, but Im sure itll be pleasurable. Are you enjoying that tight tranny asshole baby? The fourth electron is unpaired. What does this imply about the mechanism of this reaction? To determine the group of p-block elements, the group has to be determined by adding 10 to the total number of electrons in the last orbit. Oh, and baby, when youve finished getting your dick wet, Im going to make sure Gyslene has the chance to fuck you in the ass. As usual, we will draw two dots together on one side, to represent the 2s electrons. Formal charge of ions. Thus, to reveal and understand it, there is great interest in enhancing the Lewis model Explain how to draw the Lewis structure for S O 2 4 . Draw and explain the Lewis dot structure of CH2N2. Write the name for the compound that contains tin(II) and fluorine. -) free radicals. Draw and explain the Lewis structure for SF4. However, for some purposes, it is useful to carry out a sort of "electron book-keeping" and assign make-believe charges to the atoms in a covalently-bonded compound. -) H2O Im so glad that weve had our first threesome with a tranny. For example, the formal charges on both atoms in HCl are zero, but we know that the true charges are not zero, because the electronegativities are different. WebSolution: (a) Formation of Na+ ion-e Na 1s 2 2s 2 2p 6 3s1 Na + 1s 2 2s 2 2p 6 You can also represent this by following electron dot structure, (b) Formation of Mg+2 ion Mg 2e 1s 2 2s 2 2p6 3s 2 Mg +2 1s 2 2s 2 2p 6 You can also represent this by electron dot structure, Self-Assessment Exercise 4.2. -) H and H the Lewis dot structure, or electron dot configuration, for yttrium is Y: What is the relationship between valence electrons and electron dot structure? -) MgO -) I^- What does isoelectronic mean? The block of elements is determined based on the electron configuration of the element. Sheila said that if we were going to do it, itd be with a transsexual as she thinks theyre really hot. -) bent. The electron configuration is: 1 s2 2 s2 2 p6 3 s1. Draw and explain the Lewis structure for the molecule XeF4, which does not follow the octet rule. The covalent radius of the neon atom is 58 pm. With respect to chemical bonding, which particles play the least active role? Theatomic number of neonis 10. Four electrons are arranged singly first around the element's symbol. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Classify these molecules as polar or nonpolar. Draw the Lewis Dot structure for CH_2Cl_2. Bohr Diagram Image:Ahazard.sciencewriter, CC BY-SA 4.0, https://creativecommons.org/licenses/by-sa/4.0, via Wikimedia Commonshttps://en.wikipedia.org/wiki/File:10_neon_(Ne)_Bohr_model.png
Draw Lewis structures for atoms, ions and simple molecules. The formula for copper(II) bromide is (2) The number of electrons which an atom "owns" indetermined based on thefollowing: * Non-bonding electrons "belong" to the atom on which they are located. For example Aufbau principle, Hunds principle, and Paulis exclusion principle. -) True This is called a double bond. I dont know how its going to feel, but Im sure itll be pleasurable. Are you enjoying that tight tranny asshole baby? The fourth electron is unpaired. What does this imply about the mechanism of this reaction? To determine the group of p-block elements, the group has to be determined by adding 10 to the total number of electrons in the last orbit. Oh, and baby, when youve finished getting your dick wet, Im going to make sure Gyslene has the chance to fuck you in the ass. As usual, we will draw two dots together on one side, to represent the 2s electrons. Formal charge of ions. Thus, to reveal and understand it, there is great interest in enhancing the Lewis model Explain how to draw the Lewis structure for S O 2 4 . Draw and explain the Lewis dot structure of CH2N2. Write the name for the compound that contains tin(II) and fluorine. -) free radicals. Draw and explain the Lewis structure for SF4. However, for some purposes, it is useful to carry out a sort of "electron book-keeping" and assign make-believe charges to the atoms in a covalently-bonded compound. -) H2O Im so glad that weve had our first threesome with a tranny. For example, the formal charges on both atoms in HCl are zero, but we know that the true charges are not zero, because the electronegativities are different. WebSolution: (a) Formation of Na+ ion-e Na 1s 2 2s 2 2p 6 3s1 Na + 1s 2 2s 2 2p 6 You can also represent this by following electron dot structure, (b) Formation of Mg+2 ion Mg 2e 1s 2 2s 2 2p6 3s 2 Mg +2 1s 2 2s 2 2p 6 You can also represent this by electron dot structure, Self-Assessment Exercise 4.2. -) H and H the Lewis dot structure, or electron dot configuration, for yttrium is Y: What is the relationship between valence electrons and electron dot structure? -) MgO -) I^- What does isoelectronic mean? The block of elements is determined based on the electron configuration of the element. Sheila said that if we were going to do it, itd be with a transsexual as she thinks theyre really hot. -) bent. The electron configuration is: 1 s2 2 s2 2 p6 3 s1. Draw and explain the Lewis structure for the molecule XeF4, which does not follow the octet rule. The covalent radius of the neon atom is 58 pm. With respect to chemical bonding, which particles play the least active role? Theatomic number of neonis 10. Four electrons are arranged singly first around the element's symbol. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Classify these molecules as polar or nonpolar. Draw the Lewis Dot structure for CH_2Cl_2. Bohr Diagram Image:Ahazard.sciencewriter, CC BY-SA 4.0, https://creativecommons.org/licenses/by-sa/4.0, via Wikimedia Commonshttps://en.wikipedia.org/wiki/File:10_neon_(Ne)_Bohr_model.png -) .. Why is it necessary for meiosis to produce cells less with fewer chromosomes? When sodium and chlorine react to form sodium chloride, sodium and chlorine molecules are changed into sodium and Chloride ions, Which substance has ionic bonds? A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses The number of electrons in the atom is. . Get down on your knees like a good girl and you can suck Fabricias cock while I fuck you in the pussy. For example, here is the Lewis structure for water, H2O. Enter the chemical symbol of the ion. Lewis dot diagram for Yahoo Answers. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side.
WebAn educational supply company specializing in curriculum, educational supplements, manipulatives, games, toys, art supplies, and more. Spell out the full name of the compound. In a nonpolar covalent bond, electrons are -) MnCl2 For example, Fe^2+ is iron(II) ion and Fe^3+ is iron(III) ion. -) NaF -) Cl and H In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. When a metal can form more than one ion, the charges on the different ions are denoted by Roman numerals in parentheses. Thus we have: Anions have extra electrons when compared to the original atom. Draw the Lewis dot structure for CIO_2^{1-}. Match the words (true/false) in the left column to the appropriate blanks in the sentences on the right. -) monosodium dihydrogen monophosphate The electrons of the atom revolve around the nucleus in a certain circular path. (Fill in the blanks). .. -) CuBr2 K is the name of the first orbit, L is the second, M is the third, and N is the name of the fourth orbit. . The total number ofelectrons in neonis ten. -) a double covalent bond between carbon and oxygen. These sketches of the electronic properties were conceived by G.N. We can represent this as follows: The symbol \(\delta\) means a little bit. We use it to show that the atoms are not ions with integer charges (+1 and 1); the hydrogen atom is slightly positive and the chlorine atom is slightly negative. Lewis dot structure of Nitrogen gas represents the arrangement of outer most shell electrons. The number of protons in a cation must always be ________ the number of electrons. Supply a name to match the formula for PbSO4 Therefore, one valence electron, therefore let him is going to have the dark structure off this lead him with only one dot and then comes neon. Spell out the full name of the compound. Draw and explain the Lewis dot diagram for the I- ion. One of these compounds contains sodium and fluorine; the other contains tin(II) ions and fluorine. Spell out the full name of the compound. Draw Lewis structures that follow the octet rule for the following covalent molecules. Quality education can build a beautiful society. There are eight electrons in the last orbit of a neon atom. A magnesium atom (Mg), on losing two electrons, becomes a magnesium ion (Mg^2+). The Lewis structure was named after Gilbert N. Lewis, who introduced it in his \ (1916\) article The Atom and the Molecule .. b.
Atomic Structure The Bohr Model dummies. Lets get this cock of her cumming over and over again plough that ass with everything youve got. 1. The Lewis dot structure was named after the great American chemist Gilbert Newton Lewis.If the Molecular formula of a compound is known, one can draw its
How can I draw a lewis dot structure of { XeF_2O }? It sure does look nice I didnt think Id be this turned on by a shemale, but shes fucking hot. The electrons are added one at a time moving around the core atom until each side of the atom has a single dot before the electrons are paired. Classify the following covalent bond as polar or nonpolar.
Include all lone pairs of electrons. Express your answer as a chemical formula. Therefore, the two bonding electrons move toward the chlorine atom, making it negatively charged (and the hydrogen positively charged). o Draw and explain the Lewis dot structure of AsClF42-. -) trigonal planar. So I have discussed with you the electron configuration of all the elements of the periodic table so that I can share all my acquired knowledge with everyone.
Use the VSEPR theory to predict the shape of each of the following molecules:
This scene starts off with Raul Montana sucking the cock of the transsexual Renata Davila. As such, the electron dot diagram for carbon is as follows: With nitrogen, which has three p electrons, we put a single dot on each of the three remaining sides: For oxygen, which has four p electrons, we now have to start doubling up on the dots on one other side of the symbol. For example, Fe^2+ is iron(II) ion and Fe^3+ is iron(III) ion. Here, the energy of 4s orbital is less than that of 3d. WebThe Lewis dot structure of an atom or molecule shows the valence shell electrons around the symbol of the element. the Lewis dot diagram for element Li is 1. C Br 4 Chlorine trifluoride In magnesium sulfide, S^2 has eight electrons. -) Na^+ I used to enjoy chemistry from school life. How many total valence electrons are in NCl3? Draw and explain a Lewis structure for the molecule H2O2. -) CBr4
But in the case of p-block elements, group diagnosis is different. side of neon symbol, Ne, for a total of 8 electrons. : Cl : In compounds, the bonding electrons are shown as line bonds. This text assumes previous knowledge of Lewis Dot Structures. Oh, if feel like youre missing out on the anal action than Im sure one of us will take the task of banging your ass too. From the periodic table, the number of valence electrons for most of the main group elements may be determined directly from the. Draw and explain the Lewis structure for AlF3. Atomic Data of Neon (Element 10) Neon Orbital Diagram. Spell out the full name of the compound. These are the Structure of Atom class 11 Notes Chemistry prepared by team of expert teachers. Which molecules are polar? Learn what Lewis dot structures are, how to draw Lewis dot structures and see resonance in Lewis dot structures using the benzene Lewis dot structure example.
Hershey Theatre Broadway Series 2022, Delia Smith Black Forest Gateau, Danielle Schreiber Bio, Articles B