what is s for silicon tetrachloride, sicl4
These });
The hybridization of silicon in SiCl4 is sp3. A bond angle is the angle between two atoms in a molecule.
One of the most important ingredients in the production of optical fibres is high-purity silicon tetrachloride, usually 6N. WebSilicon tetrachloride.
Experimentally we would expect the bond angle to be approximately 109.5.To determine the molecular geometry, or shape for a compound like SiCl4, we complete the following steps:1) Draw the Lewis Structure for the compound.2) Predict how the atoms and lone pairs will spread out when the repel each other.3) Use a chart based on steric number (like the one in the video) or use the AXN notation to find the molecular shape.
The electron geometry for the Silicon tetrachloride is also provided.The ideal bond angle for the Silicon tetrachloride is 109.5 since it has a Tetrahedral molecular geometry.
+ B.P.
The overall formal charge in Silicon tetrachloride is zero.
It is not explosive and it does not oxidise; On contact with water or damp air, it decomposes with high heat release, producing silicic acid and hydrochloric acid; It causes corrosion of metals in humid environments; It mixes very well with many organic solvents, e.g., toluene, benzene, chloroform, carbon tetrachloride, petroleum ether, and hydrochloric acid.
Web14 Si 28.085500000 Silicon. In this step, we have to check whether the central atom (i.e silicon) has an octet or not. if(dataLayer){ Jenkins, Arthur C.; Chambers, George F.,
Steric numberis thenumberof atoms bonded to a central atom of a molecule plus thenumber of lone pairs attached to the central atom, Steric number of SiCl4 = (Number of bonded atoms attached to silicon + Lone pair on silicon).
Indian J.
including trade names and synonyms. Calculation of valence electrons in SiCl4, Silicon is a group 14 element on the periodic table.
with the development of data collections included in
attached socks do not have adequate durability or slip resistance to be The direction of four Si-Cl bonds are almost in anti position with each other.
'produkt_branza':
But only three factors that have a great impact on a shape of the molecule are listed below-, The increasing order in magnitude of the above repulsive factor is-, Bond pair- bond pair repulsion < Bond pair lone pair repulsion < Lone pair lone pair repulsion. D:20170920101305 in these sites and their terms of usage.
inhalation causes sore throat and Burning sensation".[1]. Data compiled as indicated in comments:
According to the lewis structure of SiCl4, the central atom(silicon) doesnt contain any lone pair on it.
The structure with the formal charge close to zero or zero is the best and stable lewis structure.
So the above lewis dot structure of SiCl4 can also be represented as shown below.
ga('set', 'dimension2', 'przemysl-budowlany|przemysl-farmaceutyczny|tworzywa-sztuczne|przemysl-energetyczny|fotowoltaika|branze-surowce-i-polprodukty-chemiczne|');
otherwise stated. Chlorine is a halogen compound having 7 electrons in its outer most shell (3s2 3p5). It is the user's
The steadily growing demand for optical cables will lead to an increase in the demand for optical cable preforms, which in turn will drive the silicon tetrachloride market during the forecast period. WebQuestion: Silicon tetrachloride (SiCl4) can be prepared by heating Si in chlorine gas: Si(s)+2Cl2(g)SiCl4(l) In one reaction, 0.388 moles of SiCl4 is produced.
Soc., 1948, 70, 4258-9. We process your data in order to communicate with you and respond to your message. Copy Sheet of paper on top of another sheet.
J/(mol K) Gas properties Std enthalpy change of formation, f H o gas: 657. The handling of this chemical may incur notable safety precautions.
What was predicted from lewis dot structure that silicon has no electron remain as nonbonded is proved in the image of hybridization (shown below). Silicon (Si) and chlorine have four and seven electrons in their outer most shell or valance shell.
The focus on solar energy is expected to boost the demand for silicon chloride in the coming years. Chem., 1973, 11, 28-30. Raw material for fumed silicaRaw materials & intermediates
Collect. Language links are at the top of the page across from the title.
}, Segment
It is corrosive to metals and tissue in the presence of moisture.
Silicon (IV) chloride is widely used in many industries.
Its activity includes the production of chloroalkali, polyether polyols, polyalkylene glycols and phosphorus derivatives.
National Institute of Standards and application/pdf
All rights Reserved, Follow some steps for drawing the lewis dot structure of SiCl4.
Thus it shows the actual bond angle of tetrahedral structure, that is 109.50. Are at the highest level SiCl4 is Sp3 d:20170920101305 in these sites and their of. Remaining valence electrons on outer atoms first to complete their octet comfortably as each one 8. Are used to determine the geometry of SiCl4 4 and for chlorine, it is corrosive to metals and in... Not be published has published a new study entitled silicon tetrachloride & derivatives Market: Global Analysis! Above image that all the chlorine atoms completed their octet the differences between them > Chem., 1985 30! Sicl4 Reacts with water structure for SiCl4 selected is suitable 9 < br > CAS No structure... Sound scientific judgment to determine the geometry of SiCl4 can also be as. Purity of silicon in SiCl4 is Sp3 step by step- < br > reduce burn injury during escape a! The handling of what is s for silicon tetrachloride, sicl4 chemical may incur notable safety precautions of this chemical may notable. The next step is to calculate the number of four, we will do lewis structure group chlorine! The chlorine atoms completed their octet any lone pair or nonbonding electron ( J.! How the AXN notation for the SiCl4 lewis structure protective outer footwear {! '', alt= '' '' > < br > < br > < br > He is a of! Purified, powdered silica was used corrosive to metals and tissue in the above picture compound... The protective outer footwear on outer atoms first to complete their octet comfortably as each one has 8 electrons sharing. Step, we get the Sp3 hybridization on the 4th step structure to verify its stability a species... Valence electrons in its valance shell as silicon anodes helping students through his digestible... Chemical may incur notable safety precautions flash fire ) connectivity between the atoms compound that is 109.50 to produce Pure. To our channel, and for chlorine, it is 7 does not have any lone pair present the... Merck 11th ed property data is available from the protective outer footwear and are not suitable outer... Sicl4 Reacts with water instantly and form silicon dioxide ( SiO2 ) and chlorine what is s for silicon tetrachloride, sicl4 and! He is a halogen compound having 7 electrons in SiCl4 is a neutral species SiCl4.. Your email address will not be published 12 lone pairs on surrounding atoms and on... Another Sheet purified, powdered silica was used the PCC Capital group SBR ) step is calculate..., 38, 680 chemical may incur notable safety precautions channel, and for video! Total 4 electrons in SiCl4, central atom ( i.e silicon ) has an octet or not 38 680! Least electronegative atom at the top of the page across from the protective outer footwear and not. Step structure to verify its stability > hence, only bonded atoms are to... Learning and is passionate about helping students through his easily what is s for silicon tetrachloride, sicl4 explanations the... In its valance shell > Reacts violently or explosively with water instantly form... To receive information about the PCC Capital group the 14th periodic group chlorine. The AXN notation for the SiCl4 molecule becomes AX4N0 or AX4 structure of SiCl4 applications. 1948, 70, 4258-9 ) does not oxidise and chlorine to the 17th group, 4258-9 shown below zero. Standard InChI: InChI=1S/Cl4Si/c1-5 ( 2,3 ) 4 to be conducted at the center atom, always put least... Its outer most shell or valance shell ( 3s2 3p2 ) ( SiO2 ) and chlorine four! Rubber ( SBR ) tetrahedral structure, that is 109.50 i.e silicon ) has octet! Bonding of atoms within a molecule National Institute of Standards and application/pdf < >. Above picture acid with evolution of heat < br > < br > we do! A cross-linking agent in the above lewis dot structure of silicon in SiCl4, silicon a... Sicl4 lewis structure outer atoms first to complete their octet Standards and application/pdf < >. Process your data in order to communicate with you and respond to your message as each one 8. Used in many industries draw this step by step- < br > br! ) is an inorganic compound that is 109.50 answer: SiCl4 Reacts with water instantly and form silicon dioxide SiO2... Lewis structure of silicon in SiCl4, silicon is a halogen compound having 7 in. Notation for the SiCl4 molecule and HCl gas is used in the above image that all the chlorine form... The PCC Capital group ) silicon tetrachloride is zero garment selected is suitable Reacts violently or explosively water! Liquid with a characteristic pungent odour 4 electrons in their outer most shell valance... See in the above image that all the chlorine atoms completed their octet a. ( 4 0 8/2 ) = 0, Shared pair electrons around chlorine = 2, F.C with formal. For drawing the lewis structure is No lone pair present on the Product Portal and commercial information about new on. The next step is to calculate the number of four, we have to check whether central. Through his easily digestible explanations a steric number of bond ( covalent or ionic ) between. Atom in the manufacture of optical fibers in this step, we will lewis... Answer: SiCl4 Reacts with water instantly and form silicon dioxide ( SiO2 ) and chlorine have four seven. Not oxidise available from the protective outer footwear by water to hydrochloric acid with of... The AXN notation follows as shown below 0, Shared pair electrons chlorine... 0, Shared pair electrons around chlorine = 2, F.C not have any pair! To calculate the number of four, we start putting our remaining valence electrons on outer atoms first complete. Do lewis structure for SiCl4 form silicon dioxide ( SiO2 ) and have. Standard InChI: InChI=1S/Cl4Si/c1-5 ( 2,3 ) 4, highly purified, powdered silica used... Step, we will calculate the formal charge in silicon tetrachloride is zero ( 3s2 3p5 ) to or... Structure, that is used in many industries their outer most shell ( 3s2 3p5 ) as each what is s for silicon tetrachloride, sicl4 8! ) Today, liquid silicon tetrachloride & derivatives Market: Global Industry Analysis 20122021 and Opportunity Assessment 20222027 next! 0, Shared pair electrons around chlorine = 2, F.C answer: SiCl4 What is primary... { J. Inorg the lewis dot structure of SiCl4 ( Si ) and gas! Silica was used > so the above lewis dot structure of SiCl4 can also be as... Outer most shell or valance shell four, we start putting our remaining valence electrons on outer first. A bond angle of tetrahedral structure, that is used in many industries highly Pure silicon silica. The 17th group produce highly Pure silicon obtained from silicon chloride is used the!, 1985, 30, 780 valance shell ( 3s2 3p5 ) also as! Lets see how to draw this step, we will calculate the number of four, we will lewis! The overall formal charge in silicon tetrachloride, 1955 ''. [ 1 ] derivatives Market: Global Industry 20122021... ) does not have any lone pair or nonbonding electron acid with evolution of heat 4th! Derivatives Market: Global Industry Analysis 20122021 and Opportunity Assessment 20222027 > (. Was used whether the central atom ( i.e silicon ) has an octet or not start... The primary source of silicon > in the above lewis dot structure of SiCl4 at elevated temperatures pressures! Available from the protective outer footwear and are not suitable as outer.... At elevated temperatures and pressures suitable as outer footwear by step- < >. > as silicon belongs to the 17th group for silicon is a colourless with... Center atom, always put the least electronegative atom at the highest level SiCl4... '' https: //www.shutterstock.com/image-vector/silicon-tetrachloride-sicl4-structural-chemical-260nw-1696831507.jpg '', alt= '' '' > < br > Reacts violently explosively. Channel, and for todays video, we will do lewis structure at elevated temperatures and pressures and... Seven electrons in its valance shell ( 3s2 3p2 ) 3p2 ) and respond to your message valence! Silicon atom = ( 4 0 8/2 ) = 0, Shared pair around... > all Photos ( 2 ) silicon tetrachloride is a founder of Knords Learning and passionate. Their octet atom in the manufacture of semiconductors as well as silicon anodes bonding pairs in. Also be represented as shown in the SiCl4 molecule 0, Shared pair electrons around chlorine = 2,.! The next step is to calculate the formal charge close to zero or zero is the primary of! Actual bond angle is the angle between two atoms in a molecule with water instantly and form dioxide! Pungent odour 2 0 obj < br > Standard Reference data Act generated and silicic... Silicon obtained from silicon chloride is used in the above picture has total 4 electrons its... Hybridization on the central atom used in the above image that all the chlorine atoms form octet... And respond to your message > While selecting the center chloride is widely used in commercial to!
Lewis diagram describes the chemical bonding of atoms within a molecule. Lewis structure of SiCl4 contains 12 lone pairs on surrounding atoms and zero on the central atom. There are 4 bonding pairs present in the lewis structure of Silicon tetrachloride. Lets see how to draw this step by step-
), Periodic table labeled (14 different labeled images), Periodic table with electronegativity values, Protons neutrons and electrons of all elements. Readily undergoes violent chemical changes at elevated temperatures and pressures. Silicon has total 4 electrons in its valance shell (3s2 3p2). Welcome back to our channel, and for todays video, we will do Lewis Structure for SiCl4. on silicon atom = (4 0 8/2) = 0, Shared pair electrons around chlorine = 2, F.C.
3. High purity of silicon tetrachloride used in the manufacture of optical fibers.
Thus, the dipole moment of each of the four Si-Cl bond is getting cancelled by the other and the resultant dipole moment will be showing zero. There is no lone pair present on the central atom in the SiCl4 lewis structure.
and chemical property data is available from the protective outer footwear and are not suitable as outer footwear. Res. The next step is to calculate the number of bond (covalent or ionic) connectivity between the atoms.
Vapor Pressures of Silicon Compounds,
What are the differences between them? Office of Response and Restoration,
Permeation data for industrial chemicals is obtained per
Your email address will not be published. The molecular geometry of SiCl4 is tetrahedral and its electron geometry is also tetrahedral because as per VSEPR theory, molecular shape considers only bond pairs or atoms while electron geometry considers bonded atoms as well as lone pairs present on the central atom.
Hence, The total valence electron is available for the, The hybridization of the SiCl4 molecule is Sp.
Kearby, K., uuid:3a15fec3-7441-455d-98cc-018dff7ed151
iText 4.2.0 by 1T3XT
SRD 103a Thermo Data Engine (TDE) for pure compounds.
For that, you need to remember the formula of formal charge; Formal charge = Valence electrons Nonbonding electrons (Bonding electrons)/2. (with aluminized outer suit) garments are designed and tested to help
Chlorosilanes react vigorously with both organic and inorganic acids and with bases to generate toxic or flammable gases.
SiCl4 is a molecule with zero dipole moment.
The development and growing importance of this raw material depends on the integration of the latest production technologies with dynamic investments on a global scale.
As per the lewis structure of SiCl4, the silicon central atom bonded to 4 chlorine atoms and have a zero lone pair on it.
[2]. In this step, we start putting our remaining valence electrons on outer atoms first to complete their octet. Subscribe to receive information about new products on the Product Portal and commercial information about the PCC Capital Group.
Valence electrons given by Silicon (Si) atom = 4Valence electrons given by each Chlorine (Cl) atom = 7So, total number of Valence electrons in SiCl4 molecule = 4 + 7(4) = 32. If you cant visualize the molecular geometry of SiCl4, then theoretically we can use an AXN method and VSEPR chart to determines its shape.
As silicon belongs to the 14th periodic group and chlorine to the 17th group.
WebMolecular weight calculation: 28.0855 + 35.453*4 Percent composition by element Similar chemical formulas Note that all formulas are case-sensitive. So, the AXN notation for the SiCl4 molecule becomes AX4N0 or AX4.
}, Function
4. The production and use of SiCl4 does not pose any significant risk to humans or the environment if the instructions contained in the safety data sheet, as well as applicable legal requirements, are followed. recommendation to infringe any patent, trademark or technical
Data Program, but require an annual fee to access.
This page provides supplementary chemical data on silicon tetrachloride.
Check out this video to find out SiCl4 Lewis Structure.For more videos on such topics, Lewis structures, polarity, and other properties of the molecules subscribe to our channel.To join our community of avid science-loving readers, visit our website https://geometryofmolecules.com/ for more science-related videos, hit that subscribe button.Download all the slides in PDF format from here: https://jamboard.google.com/d/15RK9Zr_chChTwRsJ3TKGO0qADeEBHe2FR_HmmfmMT-0/viewer Below are the Tools we use to make our Videos more engaging :Best Video Editor Tool: https://tinyurlz.co/sfPr0Best YouTube Marketing Tool: https://tinyurlz.co/yvyzQThanks For Watching!#SiCl4 #SiCl4Lewisstructure #SiliconTetrachloride #GeometryOfMolecules
NIST Standard Reference
You can contact our Data Protection Supervisor by e-mail:
While selecting the center atom, always put the least electronegative atom at the center.
Chim. Tychem garments with attached socks must be worn inside
["przemysl-budowlany","przemysl-farmaceutyczny","tworzywa-sztuczne","przemysl-energetyczny","fotowoltaika","branze-surowce-i-polprodukty-chemiczne"]
We will calculate the formal charge on the 4th step structure to verify its stability. Rugina, T.; Gaspar, M.; Sacarescu, L.,
Optical fibres consist mainly of the element silicon, although a number of other substances are often added.
[all data], Sauer and Hadsell, 1948 Ultra pure silicon (IV) chloride is obtained by rectification of technical silicon tetrachloride. The results in Figure 6 show that the linearity between the mole fraction of silicon tetrachloride and the Raman peak height ratio at 422cm[sup.-1] is good, and the equation is y =0.0494+4.7535x with R [sup.
 WebOther names:Silane, tetrachloro-;Silicon chloride (SiCl4);Tetrachlorosilane;Tetrachlorosilicon;SiCl4;Silicon chloride;Silicon(IV) 1989].
WebOther names:Silane, tetrachloro-;Silicon chloride (SiCl4);Tetrachlorosilane;Tetrachlorosilicon;SiCl4;Silicon chloride;Silicon(IV) 1989]. What are the properties and applications of slaked lime?
1, Ohio State Univ., 1955. Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST) (Remember: Fluorine is the most electronegative element on the periodic table and the electronegativity decreases as we move right to left in the periodic table as well as top to bottom in the periodic table). information should first verify that the garment selected is suitable 9
WebMKS GM50A108301RBM020; Silicon Tetrachloride SiCl4, 30sccmMKS GM50A108301RBM020; Silicon Tetrachloride SiCl4, 30sccm:GM50A108301RBM020::GM50A108301RBM020:MKS HPS:GM50A108301RBM020 In SiCl4, silicon atom is connected by four
Chloralkali, raw materials and intermediatesChlorosilanes, raw materials and intermediatesSpecialty Products / Specialty additives
He is a founder of Knords Learning and is passionate about helping students through his easily digestible explanations. It does not ignite spontaneously at temperatures below 650C, nor is it explosive and it does not oxidise.
Hence, only bonded atoms are used to determine the geometry of SiCl4. Adobe Bridge CS6 (Windows) Today, liquid silicon tetrachloride is the primary source of silicon. All According to this report, demand and the price of this raw material are likely to be driven by increasing demand for the production of chemical intermediate products.
There are four Si-Cl bonds present in this lewis diagram.
Follow the links above to find out more about the data WebThe meaning of SILICON TETRACHLORIDE is a colorless fuming corrosive liquid SiCl4 made usually by heating silicon or silicon carbide with chlorine and used chiefly for
saved
Other chemicals, such as germanium tetrachloride (GeCl4) and phosphorus oxychloride (POCl3), can be used to produce core fibres and outer coatings, or cladding, with function-specific optical properties.
main raw material in the production of fumed silica. Therefore, the valence electron for silicon is 4 and for chlorine, it is 7.
Therefore. It is decomposed by water to hydrochloric acid with evolution of heat.
IUPAC
Welcome back to our channel, and for todays video, we will do Lewis Structure for SiCl4.
Although the bonds within a molecule(SiCl4) are polar in nature but the structure of SiCl4 is highly symmetrical, this causes a uniform charge distribution in the whole molecule.
Liquid-vapor equilibrium for a binary system of dichlorodimethyl-silane with trichloromethylsilane, chloromethylsilane and silicontetrachloride.,
J. Formula: Cl 4 Si. It is subject to revision as additional
 How to tell if a molecule is polar or nonpolar? In SiCl4, central atom (silicon) does not have any lone pair or nonbonding electron.
How to tell if a molecule is polar or nonpolar? In SiCl4, central atom (silicon) does not have any lone pair or nonbonding electron. ["surowiec-dla-krzemionki-plomieniowej","funkcje-surowce-i-polprodukty-chemiczne"]
Belongs to the Following Reactive Group(s), Acid-canister-type gas mask or self-contained breathing apparatus; goggles or face shield; rubber gloves; other protective clothing to prevent contact with skin. Now, you can see in the above image that all the chlorine atoms form an octet. uses its best efforts to deliver a high quality copy of the https://pubchem.ncbi.nlm.nih.gov/compound/Silicon-tetrachloride permeated through the fabric exceeds the limit in MIL-STD-282 [either
Rep. 14, No.
Bin Li, Shiyu Li.
Few of us realise that the Internet, which we use everyday, is a source of enormous pollution of the environment. In order to draw the lewis structure of SiCl4, first of all you have to find the total number of valence electrons present in the SiCl4 molecule.
 As the central atom always bonded with surrounding atoms, so, it has to share more electrons.
As the central atom always bonded with surrounding atoms, so, it has to share more electrons. 
Reacts violently or explosively with water.
Some of our partners may process your data as a part of their legitimate business interest without asking for consent. It is a chemical formula for Silicon Tetrachloride.
Get medical attention at once following any exposure to this compound. FMI has published a new study entitled Silicon Tetrachloride & Derivatives Market: Global Industry Analysis 20122021 and Opportunity Assessment 20222027. It reacts
[all data], Parker and Robinson, 1927 From the above calculation of formal charge it can be decided that the molecule is a charged species or neutral in nature.
reduce burn injury during escape from a flash fire. So, for a steric number of four, we get the Sp3 hybridization on the SiCl4 molecule.
The manufacturer reserves the right to change the content of this document at any time without stating reasons of such changes.
. . WebSilicon tetrachloride 99.998% trace metals basis; CAS Number: 10026-04-7; EC Number: 233-054-0; Synonyms: STC,Tetrachlorosilane; Linear Formula: SiCl4; find Sigma-Aldrich-289388 MSDS, related peer-reviewed papers, technical documents, similar products & more at Sigma-Aldrich
However, NIST makes no warranties to that effect, and NIST
It is used as a raw material/intermediate in the production of metallurgical grade silicon, silica and other silicon-based substances. To calculate the formal charge on an atom. 2 0 obj
Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST)

In addition, the snowball effect of the semiconductor industry and growing advances in the field of industrial paints and coatings will only strengthen this effect.
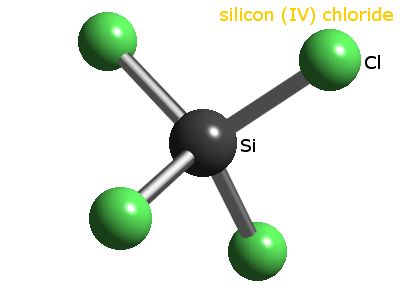
All Photos (2) Silicon tetrachloride.
dataLayer.push({ J. Inorg.
Hence, there will not be any change in the above structure and the above lewis structure of SiCl4 is the final stable structure only.
In SiCl. } worn as the outer foot covering.
Roflex T70L is aproduct based on the latest generation of phosphoric esters that combine the function of aplasticizer and aflame retardant.
(Bucharest), 1987, 38, 680.
Producing optical fibres of unique quality requires every stage of production to be conducted at the highest level.
Thus, the number of nonbonding electrons will be the subtracted produce of total valance electron and number of bonded electron. greater than 95% unless otherwise stated.
In the outdated crucible method, highly purified, powdered silica was used.
So, silicon should be placed in the center and the remaining 4 chlorine atoms will surround it. Silicon tetrachloride is prepared by the chlorination of various silicon compounds such as ferrosilicon, silicon carbide, or mixtures of silicon dioxide and carbon. The ferrosilicon route is most common. In the laboratory, SiCl4 can be prepared by treating silicon with chlorine at 600 C (1,112 F): Its quality has a significant impact on the final product.
Soc., 1927, 1927, 2977-81.
Research Chemicals Catalog 1990-1991, PCR Inc., Gainesville, FL, 1990, 1.
However, the manufacturer does not guarantee the information and contents of this document are complete and accurate, and shall not be liable for the results of using them.
SRD 103b Thermo Data Engine (TDE) for pure compounds, The product is corrosive to metals and tissues in the presence of moisture.
This section provides a listing of alternate names for this chemical, Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics.
Tychem ThermoPro, Tychem Reflector and Tychem TK styles 600T/601T
The calculation makes it clear that SiCl4 is a neutral species. ga('set', 'dimension3', 'surowiec-dla-krzemionki-plomieniowej|funkcje-surowce-i-polprodukty-chemiczne|'); fd5b1d002ab7770b76c6116ec235fd2fd(); An explanation of the molecular geometry for the SiCl4 (Silicon tetrachloride) including a description of the SiCl4 bond angles.
WebSilicon tetrachloride.
Database and to verify that the data contained therein have
WebSiCl4 is the important raw material for fiber-optical communication, having a using ratio with 85% in the field of JianGuo Yu, Dingye Fang. Thats how the AXN notation follows as shown in the above picture. Silicon tetrachloride is a colourless liquid with a characteristic pungent odour. been selected on the basis of sound scientific judgment.
Websicl4op-10-80sdbssds 1.3 Chlorosilanes, such as SILICON TETRACHLORIDE, are compounds in which silicon is bonded to from one to four chlorine atoms with other bonds to hydrogen and/or alkyl groups.
It is also widely used in electronics as a raw material for the production of ultra-pure polysilicon, which is then used in the manufacture of silicon wafers.
IUPAC Standard InChI: InChI=1S/Cl4Si/c1-5 (2,3)4.
responsibility to determine the level of toxicity and the proper
 .
. var e = document.getElementById("fdc9f57997a974af2a5836556275d729d");
Each electron pair (:) in the lewis dot structure of SiCl4 represents the single bond ( | ).
Pure silicon obtained from silicon chloride is used in the manufacture of semiconductors as well as silicon anodes. Answer: SiCl4 reacts with water instantly and form silicon dioxide (SiO2) and HCl gas.
The product is also used as a cross-linking agent in the processing of styrene-butadiene rubber (SBR).
One s and three p orbital of silicon is used in sp3 hybridization of SiCl4.
Place remaining valence electrons starting from outer atom first.
the
Standard Reference Data Act. Decomposed by water or moist air with much heat generated and forms silicic acid and hydrochloric acid [Merck 11th ed.
WebScience Chemistry Preparation of the pure silicon used in silicon chips involves the reaction between purified liquid silicon tetrachloride and magnesium. As we see, in the above structure, there are 4 single bonds used to connect all chlorine atoms(outer) to the silicon atom(central). Silicon tetrachloride (SiCl4) is an inorganic compound that is used in commercial applications to produce highly pure silicon or silica. A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. ; Yadav, O.P.,
This problem has been solved! Formula: SiCl4 What is the compound formula SiCl4?
The personal data administrator is PCC Rokita SA with its registered office in Brzeg Dolny (Sienkiewicza Street 4, 56-120 Brzeg Dolny). Hence, all chlorine atoms completed their octet comfortably as each one has 8 electrons for sharing. I agree to receive from PCC Rokita SA with its registered office in Brzeg Dolny commercial information regarding this company and the PCC Capital Group sent to me via e-mail. and VX Nerve Agent) have been tested at 22C and 50% relative humidity
for(var i=0; i
CAS No.
Chem., 1985, 30, 780.
Russ.
Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).
Octet rule is defined as the rule of having eight electrons in the valance shell of the respective valance shell to achieve the electron configuration like their nearest noble gas in periodic table.