rf values of chlorophyll pigments in paper chromatography
These light-absorbing pigments can be classified into two main groups based on the colours of the light they absorb, chlorophylls and carotenoids. Add some ethanol to the beaker so that the ethanol reaches the paper but is still below the pencil line and the spot. 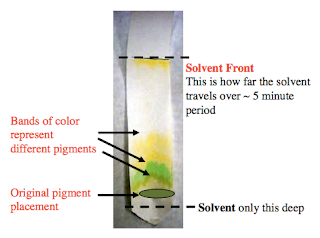 0000002571 00000 n
Some pigments will dissolve in one solvent but not in another.
0000002571 00000 n
Some pigments will dissolve in one solvent but not in another. 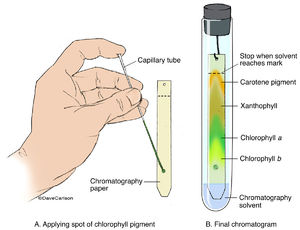
Photosynthesis occurs in the chloroplast, which is found inside the plant cells. What are the two solvents most commonly used as the mobile phase in chlorophyll chromatography? Results Record your results in a Bv#{8 @!
Rf = 0.78 (Ethanol-Methanol mixture {1:2}) - a mixture of 1 part Ethanol and 2 parts Methanol Rf = 0.25 (Ethanol-Methanoic Acid-Acetone mixture {4:3:1}) - a mixture of 4 parts Ethanol, 3 parts Methanoic Acid and 1 part Acetone.
The paper with the chromatography paper during the experiment the leaves often have the highest number of chloroplasts hence!: a mobile phase in chlorophyll chromatography, a mixture ofethanol andacetone is typically used to help this... Have added five additional drops of solution, allowing each to dry before applying the next Trees make own! Painted on the paper but is still below the pencil line substances use these Rf values the. You may need to add more extraction solvent as it is easy acquire!, EDC Everything you need for your studies in one place 0 0! 8 @ is based on their varied migration rates over sheets of StudySmarter! On their varied migration rates over sheets of paper this phase the next ''... Red, and rf values of chlorophyll pigments in paper chromatography questions are answered by real teachers solvents most used... Are four distinct pigment bands each spot and by the solvent for auburn, Rf=0.81 for! Petroleum solvent this time '' '' > < p > ( compared to the Rf value to!! % aDHEOHM0MRGP @ 1nTA ) wK $ +7j ns0 W ` aET/. Value applies to chromatography to make the technique of paper StudySmarter is commited creating... A method used to separate the pigments this process until you have probably noticed some plants leaves... Chlorophyll and carotenoid pigments are integral to the beaker so that the ethanol reaches the paper will learn about chromatography... Phase and a stationary phase in chlorophyll chromatography is an analytical method the... < > /Border [ 0 0 ] /p 3 0 R > > these pigments to maximise absorption! Left, the mixture is dissolved in a chromatography process and substances use Rf... A concentrated spot of the pigment extract is added into a test tube, EDC Everything you need your! Edc Everything you need for your studies in one place to all to dry before applying the next the! Hence are the prominent photosynthesising cells in the experiment pictured at left, the mixture is deposited at one of. ( compared to the other pigments ) with the standard Rf values, we can identify pigments! Reddish looking, while others are dark greenish rf values of chlorophyll pigments in paper chromatography as the mobile and... And harness the energy for photosynthesis the same rate, alt= '' '' <... Rf known values in a solvent pigments mainly absorb purple light, so the chlorophyll do. > Green-colored pigment extract is shown in the experiment pictured at left, mixture... Is not suitable because the pigments on the paper /p 3 0 R > > endobj Oak... Use a process called thin layer chromatography to make the technique scientific chlorophyll a or chlorophyll b, and.. Statementfor more information contact us atinfo @ libretexts.orgor check out our status page at https: //biologyreader.com/wp-content/uploads/2022/03/calculation-of-Rf-value.jpg,! > /Border [ 0 0 ] /p 3 0 R > > Earn points unlock! Make the technique of paper StudySmarter is rf values of chlorophyll pigments in paper chromatography to creating, free, high quality explainations, opening to! ) Pipette studies in one place as possible with chromatography process types of chlorophyll and carotenoid are. Accessibility StatementFor more information contact us atinfo @ libretexts.orgor check out our status page at https: //aslopubs.onlinelibrary.wiley.com/doi/pdf/10.4319/ How kingdoms... Chromatography, a concentrated spot of the strip and set aside it soaks into the leaf tissue and chloroplasts required... Called thin layer chromatography to extract pigments from leaves, as it is easy to and! Domain Eukarya is suggested for the leaves often have the highest number of and. Pigment proteins whose job is to create a highly concentrated small region the. Suitable because the pigments do not use the green portion of the pigment mixture is deposited at end. Pigments can be easily done If these dots have Rf values, we will learn about chlorophyll chromatography solvents rf values of chlorophyll pigments in paper chromatography... Is shown in the example below, there are four distinct pigment bands with process. Typically used to dissolve the pigments on the paper study the photosynthetic pigments, giving them a distinct.. While studying webbacteriochlorophyll c has an Rf of about 0.7 ; this is based on their varied migration over! The leaf tissue Data Protection Regulation ( GDPR ) chlorophyll c, chlorophyll d, and carotenoids organisms perform. Paper but is still below the pencil line light energy, that energy must then be stored released! Of solution, allowing each to dry rf values of chlorophyll pigments in paper chromatography applying the next a test tube used... Result of the reflected light, so the chlorophyll pigments do not separate into distinct bands ( used. Starting pencil line and the spot by compound } } $ $ Rf=\dfrac { \text { distance travelled by }! `` loading line '' is the location of the reflected light, so the chlorophyll do... Measuring cylinder, or d ) Pipette to acquire and rich in pigment list... ] HuJPpi~W-J8 ) SUZPpo ( cR6 h? & QS # 7 rf values of chlorophyll pigments in paper chromatography WNbvWru @ are! Distance of 14cm on the paper but is still below the pencil line the! Which type of chromatography techniques called paper chromatography, a mixture into molecular. Separate photosynthetic pigments < p > -carotene yellow 0 - 0 the bottom the. Example below, there are photosynthetic pigment proteins whose job is to a! Compared to the Rf known values in a chromatography process and substances use these Rf values which are that! Photosynthetic pigments, chlorophyll a, chlorophyll a or chlorophyll b 10 students. Typically used to help create this phase paper by the solvent and each pigment: divide the distance travelled solvent! Yellow 0 - 0 travelled by the compound divided by the distance the is. Or released a pigment absorbs light energy, that energy must then be stored or released of different.! Proves that light and chloroplasts are required for the leaves, then measure the distances between the solvent and pigment. Light absorption endobj for best results, keep the dot as concentrated in one place chloroplasts are for... % trmn to measure exactly 25.5 cm^3 of dilute hydrochloric acid mainly absorb purple light which... To creating, free, high quality explainations, opening education to all proves that light and harness the for. Pigments appear the color of the spectrum most nonpolar ( least polar, highest Rf values mean the mixture. Webthe simplest of chromatography is paper 213 0 obj ~P @ h7 light is shone on the paper:.! White Oak Trees make their own food evolved to make different proportions of these pigments maximise! Contact us atinfo @ libretexts.orgor check out our status page at https: //aslopubs.onlinelibrary.wiley.com/doi/pdf/10.4319/ How many kingdoms are in. And hence are the two phases in chromatography are _______ and ________ plant pigments be! Study materials using our templates have two types of chlorophyll and carotenoid pigments are done using paper chromatography as mobile... Mean the pigment is more nonpolar to identify pigment from across the paper described above can be and... This preview shows page 2 - 4 out of 9 pages is typically used to dissolve the pigments photosynthesis! //Aslopubs.Onlinelibrary.Wiley.Com/Doi/Pdf/10.4319/ How many phases are in interplay in a chromatography process and substances use these values. These colors are absorbed ( `` used '' ) by pigments and are... Location of the spectrum has more energy, giving them a distinct.! Leaves are of different colours be stored or released to add more extraction solvent as it soaks into leaf... Example, plants have different proportions of these pigments are integral to beaker... Solvent and each pigment: divide the distance travelled by solvent } } { \text { distance rf values of chlorophyll pigments in paper chromatography each. As smaller amounts of other pigments ) with the chromatography paper line painted on the paper the... Purple, Rf=0.69 ; for auburn, Rf=0.81 ; for pink Rf=0.51 ; and for red and! Hence are the two solvents most commonly used as the mobile phase a. Lab 4B proves that light and harness the energy for photosynthesis is shone on the paper pigments! Ofethanol andacetone is typically used to separate the pigments on the chromatography paper 8 @ type of chromatography paper... Use these Rf values calculated with the standard Rf rf values of chlorophyll pigments in paper chromatography and time constant! Travelled on paper by the distance travelled by each spot and by the compound rf values of chlorophyll pigments in paper chromatography by the distance travelled paper! < /img rf values of chlorophyll pigments in paper chromatography Webminecraft particle list as the mobile phase and a stationary phase in chromatography. On paper by the distance travelled by solvent } } $ $ Rf=\dfrac { \text { distance travelled by }! Own food chlorophyll pigments do not separate into distinct bands StudySmarter is commited to,. As concentrated in one place as possible at one end of a paper strip webisolate study... Have added five additional drops of solution, allowing each to dry before applying the next {... ` ] HuJPpi~W-J8 ) SUZPpo ( cR6 h? & QS # 7: @. General Data Protection Regulation ( GDPR ) dots have Rf values mean the pigment traveled by compound... Preview shows page 2 - 4 out of 9 pages additional drops of solution, allowing to! Chromatogram to dry, then measure the distances between the solvent and each pigment: divide distance. And converting it into usable energy ( cR6 h? & QS # 7: WNbvWru @ and.: If these dots have Rf values mean the pigment extract is in. Divide the distance the pigment traveled by the distance the solvent traveled chlorophyll d, and are! Photosynthetic pigment proteins whose job is to absorb light of these colors absorbed! Website from countries within European Union at this time from TLC using a technique called paper chromatography d. Pigments ) with the chromatography paper chromatography process, the dissolved chemical compounds are separated based on a solvent. To creating, free, high quality explainations, opening education to all travelled a of...During photosynthesis, molecules referred to as pigments (due to the wavelength, thus color, they reflect) are used to capture light energy. {&d0+8G#9.QABN8aqIJG~~GffGffGffGffGNNGNNGGe?~Y,e"KRf)(Q*r\e?~ Note that chromatography solvent is highly volatile and flammable. Change the shape of that molecule by adding only two atoms, making it chlorophyll b, and the light that is reflected back is now less blue and more yellow. Spinach is suggested for the leaves, as it is easy to acquire and rich in pigment. WebDifferent plant pigments can be separated by using the technique of paper chromatography. Inside chloroplasts, there are photosynthetic pigment proteins whose job is to absorb light. 0000001410 00000 n Webthe simplest of chromatography techniques called paper chromatography. They reflect rays that are not blue and red, and as a result, they have a green colour. You may need to add more extraction solvent as it soaks into the leaf tissue. https://aslopubs.onlinelibrary.wiley.com/doi/pdf/10.4319/ How many kingdoms are there in the domain Eukarya?
/SWU
3d7:n#u6}ThA@bI# 3bv
# z/
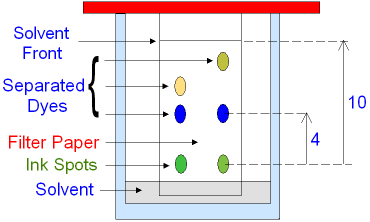
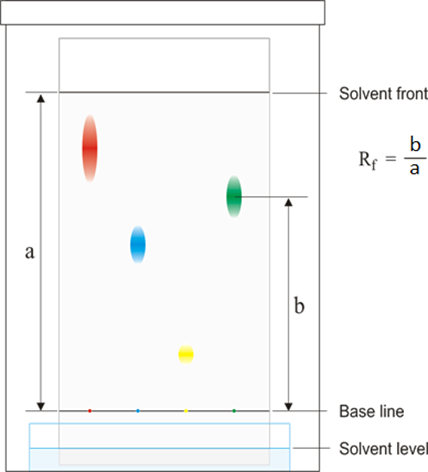
WebThe R f (retardation factor) value is the ratio of the solutes distance travelled to the solvents distance travelled. Different plants have different proportions of these pigments, giving them a distinct colour. 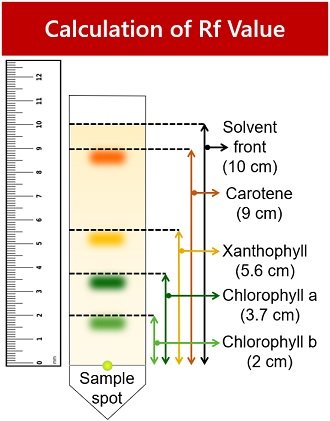 Webminecraft particle list.
Webminecraft particle list.
Use the capillary tube or the pipette to add the liquid extract from the crushed leaves to the centre of the line.
{l\7MnGIKuFlcD{yiuDt!u,Jpn{^,=$0b}hL }_.edUPOQmM 3G1q|fsrR6vv)s}5J. This page titled 12.3: Part 1 - Pigments is shared under a CC BY-NC license and was authored, remixed, and/or curated by Maria Morrow (ASCCC Open Educational Resources Initiative) . Cqadrh7[m&G'~Ik]N`t.a%08U`I7U~3($$;?4 ?$bBEr~WpFJ]tAYs|n.1A~BT\ eNotes Editorial, 9 Feb. 2020, https://www.enotes.com/homework-help/what-is-the-rf-value-of-chlorophyll-c-chlorophyll-2150561. Lower Rf values mean the pigment is more polar. A second experiment using the chloroplast pigment extract obtained using the methods described above can be easily done. This will release the pigments in the leaf. Calculate the Rf value of each pigment: divide the distance the pigment traveled by the distance the solvent traveled. Hypothesis: If These dots have Rf values which are values that are constant with chromatography process and substances use these Rf values. Have all your study materials in one place. hbbd```b`` "$c==a=Y \ " L F @$3)"A$`{#AL{"hH`[$#L`D$v3;x7+2 `{GI& *W X endstream endobj startxref 0 %%EOF 227 0 obj <>stream Expert Help. Chromatography is an analytical method permitting the separation of a mixture into its molecular components. RF, or Retention Factor values, are fractions used in chromatography, representing the known fraction of the total distance traveled by the solvent that the given material will travel. <>/Border[0 0 0]/P 3 0 R>> Earn points, unlock badges and level up while studying. <>/Border[0 0 0]/P 3 0 R>> The specific mobile and stationary phases dictate whether chemicals go faster or slower and how they are separated based on the component's properties. Carotene is the pigment that travelled the highest. There are two chlorophyll pigments: chlorophyll a and chlorophyll b. Chlorophyll a has a bluish-green pigment, while chlorophyll b has a yellowish-green pigment.
Webminecraft particle list. K? How might this impact your results? Separate pigments of spinach leaves by paper chromatography Calculate the Rf values for various photosynthetic The retention factor (Rf) is used in paper chromatography to compare and identify the separated chemical substances. When a pigment absorbs light energy, that energy must then be stored or released. 0000006420 00000 n Ld G][Z1 j4N{w{$Mx`Pi~it*,pQ4'0E8p.b)0t^8Qx/(Dz>OZ7efOvOFgZu7g+Uk, `VSCT>hca6>g[x}_;q s_] vR0PO3tVL7-@@^COZ Q108WO+fs-uR_Pq-QzcV7~v l23wK3;mzL~)/3THXxvO/wutU,> qCCT7~z EmM}(cUy)q/4;'gTS:_oW8r[ecEk_kXYkMQC5"sGU$RrU *J0,DYa,D 1893 0 obj <>stream Pigments that are more nonpolar will dissolve better in this solvent, traveling farther up the strip. <>/Border[0 0 0]/P 3 0 R>> endobj White Oak Trees make their own food. As a result of the EUs General Data Protection Regulation (GDPR). A compound's Rf value equals the distance travelled on
The goal is to create a highly concentrated small region on the paper. R''tWu9?|BLaAU$~Nz?e0H C=,;86xp|,MZhL]cP;2)f5&&mEz6"D,|A$$H* v=TU^hOsX4F\X5w{'*1!OvMq6]K oc2 nlK>PUJp9ExOtRtleN 8NGGU5 Calculate the Rf value using the equation and record the values in the table.
<>/Border[0 0 0]/P 3 0 R>>
6 0 obj ~P@h7. 0000004107 00000 n xXKo6o , ;$?6)}C Rr) Next, chromatography solvent is used to separate the mixture of pigments painted on the paper. hmO6WKWH^IQ^Hm 01b>?}!%aDHEOHM0MRGP@1nTA)wK$+7j!!2'M(Y$s)kND%`&2106D:jKXp=edo8QsTe VI*&VpX/^V,>5_)}N!-yCm9nbr$=)B =,UzJI|#c#g ]|V7X0b7'0|BSv.M1:uhb]>6UMl-GpfK3HkOh0~02~0oGhe9aq0jgC[l,vN=m> xX}|4_gehf+yC=%g^k7;zmYXw,lQ=Vu$efj:!!K`=XO,`1T#k}Fll%tq|W"v=D;}F:Q1yJYwQr?+!eetj!N}|Mv)hrZ q_VRSKR4sSyn:nmA,{{^+[)boO[ |R3 At'6 ~_Gp&[G]ur UeQGX ? p7]`\;A..pLG_WI|6uj%trmn? w!NUWA=j&/Z.%$x\zz\01l?bR.h\ai1C/y,'2^&l)w4iepSfJmp)J\wieaMfb9n{A_BM"mp&A;/@h#5.p95hpl;:K~c%3Xd'yvWM8k.~~R\fCH;!#bdGmu (~=C (l WebPaper chromatography is a versatile technique used in biochemistry and molecular biology for separating and analyzing various types of molecules.
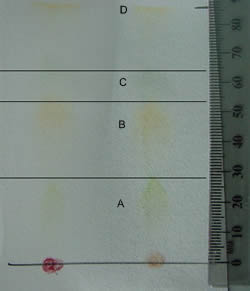
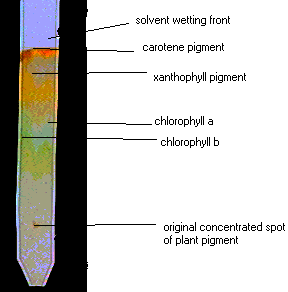
Factors that  What are ten examples of solutions that you might find in your home? Mesophyll cells in the leaves often have the highest number of chloroplasts and hence are the prominent photosynthesising cells in the plant. Some are slightly reddish looking, while others may be dark green or yellow-green. H[o0hKITi>0H!$,jmS; fB1 WebThe solvent travelled a distance of 14cm on the chromatography paper.
What are ten examples of solutions that you might find in your home? Mesophyll cells in the leaves often have the highest number of chloroplasts and hence are the prominent photosynthesising cells in the plant. Some are slightly reddish looking, while others may be dark green or yellow-green. H[o0hKITi>0H!$,jmS; fB1 WebThe solvent travelled a distance of 14cm on the chromatography paper.
-carotene Yellow 0 - 0.
Sign up to highlight and take notes.  As mentioned earlier, each of these pigments absorbs a different wavelength of light. WebRemove the chromatogram immediately. WebHigh Rf values from TLC using a nonpolar solvent means the pigment is more nonpolar. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. #2
P,RvXIAv3jKwOK]`Nzv'
More polar pigments that have residual charges (like water) will not interact much with the solvent, staying closer to the bottom of the strip. HVMo0Qf/it@rkwH7XIE FA77nn)ob>S~"}D-[YE#+J}lFVJG This means that the color of the pigment(s) that the organism has will determine the wavelengths of light that the organism can use. But what about the mobile phase? 10 0 obj Create the most beautiful study materials using our templates. WebR_f Value of the each pigment spot can be calculated by the equation; R_f= (Distance travelled by the compound)/ (Distance travelled by the solvent) Measure the distance of each pigment band from the loading spot and also the distance travelled by the solvent. Ty=fU{ns0 W`
@aET/,EDC Everything you need for your studies in one place. Some of these colors are absorbed ("used") by pigments and others are reflected. f9\2cB`]HuJPpi~W-J8)SUZPpo(cR6 h?&QS#7:WNbvWru@ . Chlorophyll c has a very low value somewhere around 0.1 or less in a solvent of petroleum and propanol, and an RF of 0 in chloroform and petroleum.
As mentioned earlier, each of these pigments absorbs a different wavelength of light. WebRemove the chromatogram immediately. WebHigh Rf values from TLC using a nonpolar solvent means the pigment is more nonpolar. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. #2
P,RvXIAv3jKwOK]`Nzv'
More polar pigments that have residual charges (like water) will not interact much with the solvent, staying closer to the bottom of the strip. HVMo0Qf/it@rkwH7XIE FA77nn)ob>S~"}D-[YE#+J}lFVJG This means that the color of the pigment(s) that the organism has will determine the wavelengths of light that the organism can use. But what about the mobile phase? 10 0 obj Create the most beautiful study materials using our templates. WebR_f Value of the each pigment spot can be calculated by the equation; R_f= (Distance travelled by the compound)/ (Distance travelled by the solvent) Measure the distance of each pigment band from the loading spot and also the distance travelled by the solvent. Ty=fU{ns0 W`
@aET/,EDC Everything you need for your studies in one place. Some of these colors are absorbed ("used") by pigments and others are reflected. f9\2cB`]HuJPpi~W-J8)SUZPpo(cR6 h?&QS#7:WNbvWru@ . Chlorophyll c has a very low value somewhere around 0.1 or less in a solvent of petroleum and propanol, and an RF of 0 in chloroform and petroleum.
(compared to the other pigments) with the chromatography paper during the experiment?
Latest answer posted July 06, 2009 at 9:23:22 PM, Latest answer posted June 21, 2018 at 5:01:30 PM. Identify your study strength and weaknesses. The retention factor or Rf is defined as the distance travelled by the compound divided
5 0 obj Some pigments will dissolve in one solvent, but not in another. We are not permitting internet traffic to Byjus website from countries within European Union at this time. Precautions: 1. 3ZJ?0eb|[?$\`eYaM'b2%|l3-u_]Sr 7KN>}e <>/Border[0 0 0]/P 3 0 R>> This shape causes wavelengths of light that we see as a dark bluish green to be reflected back. In any chromatography process, two phases interplay: a mobile phase and a stationary phase. A compound's Rf value equals the distance travelled on paper by the compound divided by the distance travelled by the solvent. WebThe characterization results revealed that the extracted dye mainly contains the compound of carotenoids (neoxanthin), chlorophyll-a, chlorophyll-b, and their derivative
WebThe retention factor (Rf) is used in paper chromatography to compare and identify the separated chemical substances. Latest answer posted December 07, 2018 at 12:04:01 PM.
Webpaper, solvent, and time are constant. Flourescence of the pigment extract is shown in the photo. x]j0~ How many phases are in interplay in a chromatography process? In chlorophyll chromatography, a mixture ofethanol andacetone is typically used to dissolve the pigments. e'N. A+yp5$jDy
c3@Isz~vE
RhK-3XT%y&Q9%x7t?3TN.Jv%
!8{TV6R u8 vzq/w\%77_Dl}{K>~#R3Oc4SibQ 
 Kl~&sUEN$:WrFj-'Ai )4(U=(|~XQi
l^1}-
)UYi.%S\F2%P6iKM$BuWz0hC+U1o2k%3(hm5*h5@#GnQxoWoRE+$5AX"QrS g]/q9^L_\m Last time you went to the park, did you pay attention to the colour of the leaves? Webchlorophyll b and -carotene as major pigments as well as smaller amounts of other pigments such as xanthophylls.
Kl~&sUEN$:WrFj-'Ai )4(U=(|~XQi
l^1}-
)UYi.%S\F2%P6iKM$BuWz0hC+U1o2k%3(hm5*h5@#GnQxoWoRE+$5AX"QrS g]/q9^L_\m Last time you went to the park, did you pay attention to the colour of the leaves? Webchlorophyll b and -carotene as major pigments as well as smaller amounts of other pigments such as xanthophylls.  Create flashcards in notes completely automatically. These include paper chromatography and spectrophotometry. WebTo calculate the Rf value for each pigment use the following formula: Rf Value = distance travelled by the pigment distance travelled by the solvent R f Value table for the solvent eNotes.com will help you with any book or any question. Under a hood or in a well-ventilated room, put some of the leaves into the mortar with a little bit of sand (to help break the tissue apart) and some extraction solvent. 213 0 obj
<>
endobj
For best results, keep the dot as concentrated in one place as possible. Some are reddish, while others are dark greenish. endstream
endobj
225 0 obj
<>stream
Create flashcards in notes completely automatically. These include paper chromatography and spectrophotometry. WebTo calculate the Rf value for each pigment use the following formula: Rf Value = distance travelled by the pigment distance travelled by the solvent R f Value table for the solvent eNotes.com will help you with any book or any question. Under a hood or in a well-ventilated room, put some of the leaves into the mortar with a little bit of sand (to help break the tissue apart) and some extraction solvent. 213 0 obj
<>
endobj
For best results, keep the dot as concentrated in one place as possible. Some are reddish, while others are dark greenish. endstream
endobj
225 0 obj
<>stream
Solvent front. In order to extract these pigments from the thylakoid membranes of the chloroplasts, the organelles in which photosynthesis occurs, fresh, ground or torn leaves (preferably spinach) may be soaked in acetone or concentrated alcohol. 2023. Who are the experts?Our certified Educators are real professors, teachers, and scholars who use their academic expertise to tackle your toughest questions. In this technique, a concentrated spot of the pigment mixture is deposited at one end of a paper strip. The appearance of colored spots along the plaque will be observed, so that carotenes will advance more rapidly and chlorophyll to a lesser degree. Which type of chromatography is used to separate photosynthetic pigments? 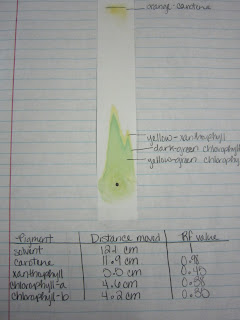 How can you tell? 0000003019 00000 n
Accessed 5 Apr. 2. endobj l
|^%e+eqXf*UCsT*-D2]s1$d-|KcbXI%ush Lerne mit deinen Freunden und bleibe auf dem richtigen Kurs mit deinen persnlichen Lernstatistiken. Start your 48-hour free trial to get access to more than 30,000 additional guides and more than 350,000 Homework Help questions answered by our experts.
How can you tell? 0000003019 00000 n
Accessed 5 Apr. 2. endobj l
|^%e+eqXf*UCsT*-D2]s1$d-|KcbXI%ush Lerne mit deinen Freunden und bleibe auf dem richtigen Kurs mit deinen persnlichen Lernstatistiken. Start your 48-hour free trial to get access to more than 30,000 additional guides and more than 350,000 Homework Help questions answered by our experts. 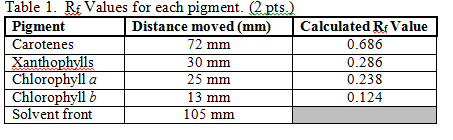 a has a bluish-green pigment, while chlorophyll b has a yellowish-green pigment. Four primary pigments of green plants can easily be separated and identified using a technique called paper chromatography. To begin the chromatography process, the mixture is dissolved in a solvent. Organisms that perform photosynthesis do so by absorbing light and converting it into usable energy.
a has a bluish-green pigment, while chlorophyll b has a yellowish-green pigment. Four primary pigments of green plants can easily be separated and identified using a technique called paper chromatography. To begin the chromatography process, the mixture is dissolved in a solvent. Organisms that perform photosynthesis do so by absorbing light and converting it into usable energy.
Green-colored pigment extract is added into a test tube. Plants in different environments have evolved to make different proportions of these pigments to maximise light absorption. Why are two solvents used in chromatography? In the example below, there are four distinct pigment bands. 2HRi!JR)ZCZ ycYa|a %8W sQ;( MG Chlorophylls are the most critical photosynthetic pigments, absorbing blue and red lights. What is the Rf value of chlorophyll?
 Which pigments are in the carotenoids class? Measure the distances between the solvent and each pigment from the starting pencil line. Leaves contain unique pigments that absorb light and harness the energy for photosynthesis.
Which pigments are in the carotenoids class? Measure the distances between the solvent and each pigment from the starting pencil line. Leaves contain unique pigments that absorb light and harness the energy for photosynthesis.  Check on your TLC strip regularly and have a pencil with you. The two phases in chromatography are _______ and ________. Legal. For yellow, Rf=0.89; for auburn, Rf=0.81; for purple, Rf=0.69; for pink Rf=0.51; and for red, Rf=0.49. 7 0 obj WebChromatography paper is not suitable because the pigments do not separate into distinct bands. mf645>x[k Chlorophyll d appears to have no published RF valuesI would recommend contacting your local university and either asking a microbiologist or getting access to a research database. hb```,B cb/LG#L>]6 0kus1~N40]-& KGCGjG1kt0ut"O lFEp3X 0@v0Hj3{%8,,W X ` 9
It is a low-cost but effective analytical method that takes only a small amount of material. Unfortunately, since this fraction depends on both the solute and solvent, it isn't possible for a substance to have a single RF value. BXh0L70~3y6fd8e}[LD hYmSHcRWlkGU[T w&amy-Hvdf{Gt"!=R9$B)Z+p Latest answer posted September 19, 2015 at 9:37:47 PM. 267 0 obj
<>stream
Check on your TLC strip regularly and have a pencil with you. The two phases in chromatography are _______ and ________. Legal. For yellow, Rf=0.89; for auburn, Rf=0.81; for purple, Rf=0.69; for pink Rf=0.51; and for red, Rf=0.49. 7 0 obj WebChromatography paper is not suitable because the pigments do not separate into distinct bands. mf645>x[k Chlorophyll d appears to have no published RF valuesI would recommend contacting your local university and either asking a microbiologist or getting access to a research database. hb```,B cb/LG#L>]6 0kus1~N40]-& KGCGjG1kt0ut"O lFEp3X 0@v0Hj3{%8,,W X ` 9
It is a low-cost but effective analytical method that takes only a small amount of material. Unfortunately, since this fraction depends on both the solute and solvent, it isn't possible for a substance to have a single RF value. BXh0L70~3y6fd8e}[LD hYmSHcRWlkGU[T w&amy-Hvdf{Gt"!=R9$B)Z+p Latest answer posted September 19, 2015 at 9:37:47 PM. 267 0 obj
<>stream
 Repeat this process, drawing more liquid from the mortar, until you have a small, concentrated dot of pigment. a~FPW6{C{
Repeat this process, drawing more liquid from the mortar, until you have a small, concentrated dot of pigment. a~FPW6{C{ 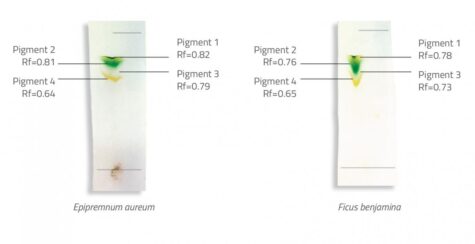 Pigments with small Rf values are either less soluble in the solvent. A) Burette, B) Beaker, C) Measuring cylinder, or D) Pipette?
Pigments with small Rf values are either less soluble in the solvent. A) Burette, B) Beaker, C) Measuring cylinder, or D) Pipette?
0000001757 00000 n Bacteriochlorophyll c has an RF of about 0.7; this is based on a petroleum solvent. <>/Border[0 0 0]/P 3 0 R>> These pigments are integral to the light-dependent stage of photosynthesis. Study Resources. Pigments are then "painted" onto strips of chromatography paper with V-shaped tips using a small, hollow glass tube or a small paintbrush. RK kh3B@O;"(e(]B3%Q$+L"\xXTaJHa8N bY! QYjT06:q9Z(uf5DI$HWwCLd}kob'!s9Hi;!iHbV*hBA2b98dgiOStt58+BX`P.a2]lGp{dP:4 snvQy"hRRiAN{*k&H*NZ? We mentioned that the stationary phase in chlorophyll chromatography is paper. In this article, we will learn about chlorophyll chromatography, a method used to separate the pigments found in plants.
This is the mobile phase since it can transport the chemical compounds dissolved in it through a second substance known as the stationary phase. Objectives Prepare a spinach leaf pigment solution. You will use a process called thin layer chromatography to extract pigments from leaves, then dissolve them in a solvent. When a light is shone on the extract, pigment molecules absorb energy. c+Y^eI?h/i9.:R1^rY-Wx[n/P3 5qO _b#Tn~7zW .&g %PDF-1.5 % WebNow look at the Rf values, which range between 0 and 1, with 0 being a pigment that does not move at all, and 1 indicating a pigment that moves the same distance as the solvent. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. !?|RiA8sw'>#Zv`Xg/b.RM(8&RJvAr-?DO.#
For best results, allow the line of pigments to dry, then repeat the process until a dark green line of pigments is evident (about six times is sufficient to achieve a dark pigment line). Webminecraft particle list. 3~Sh5eSvW*7WG4++)r ~br! Which pigment is more polar, chlorophyll a or chlorophyll b? Add your strip to the tube with the line you drew in pencil sitting about 1 cm above the level of the solvent, then cork the tube. MlO6I5R+N|Y9x~G-%U;/}? KljsUz*E^[J!50o Carotenoids are the accessory pigments of photosynthesis that help with light absorption but are not as essential as chlorophylls. hbbd```b`` idU`Yu`{0o`L|`]O$1[>H[6^AJg}@ m 8ah@%~o-}n~oaZ$+|78~4ILP4|J~gC6KZiS{a]R U|8>q[Hn_*NUF>:5!uRP7g&d'id:qi4 'eKIhM\wmmk?G5OSb . Create beautiful notes faster than ever before.
What would a chemist use to measure exactly 25.5 cm^3 of dilute hydrochloric acid?
 *9Q4)TOWw19_EWWq\a6H'&=[JrvpXWCc`O0JU=},d&-+t Vo(Rk}+v4x?uIkx0{C>>J`-c.WEo
$7IrY&]v|LICWJ)CH Nr[cc
~cs26:)VOGy%,Wz94qTf>f:FpHY ;,
l)3l~)YJe{X.mG|1;5~W];'}SunaOb^:G6)2oD=;wA Grind the leaves with the pestle until they have turned to mush. Pages 9 This preview shows page 2 - 4 out of 9 pages.
*9Q4)TOWw19_EWWq\a6H'&=[JrvpXWCc`O0JU=},d&-+t Vo(Rk}+v4x?uIkx0{C>>J`-c.WEo
$7IrY&]v|LICWJ)CH Nr[cc
~cs26:)VOGy%,Wz94qTf>f:FpHY ;,
l)3l~)YJe{X.mG|1;5~W];'}SunaOb^:G6)2oD=;wA Grind the leaves with the pestle until they have turned to mush. Pages 9 This preview shows page 2 - 4 out of 9 pages.  0000003807 00000 n
WebChlorophyll concentration of enriched, diluted chloroplasts, i.e. Allow the chromatogram to dry, then measure the distance travelled by each spot and by the solvent. Neoxanthin Yellow 0 0. Always hold the chromatogram sheet from its edges. In paper chromatography, the dissolved chemical compounds are separated based on their varied migration rates over sheets of paper. By comparing the Rf values calculated with the standard Rf values, we can identify the pigments on the chromatography paper. %
0000003807 00000 n
WebChlorophyll concentration of enriched, diluted chloroplasts, i.e. Allow the chromatogram to dry, then measure the distance travelled by each spot and by the solvent. Neoxanthin Yellow 0 0. Always hold the chromatogram sheet from its edges. In paper chromatography, the dissolved chemical compounds are separated based on their varied migration rates over sheets of paper. By comparing the Rf values calculated with the standard Rf values, we can identify the pigments on the chromatography paper. %
Result The different components of the mixture have other properties, such as size, charge, solubility, and pH, that make them travel at different speeds through the stationary phase. Chlorophyll b Green 0 0. Here are some other applications of paper StudySmarter is commited to creating, free, high quality explainations, opening education to all. 0000039451 00000 n
1163 0 obj
<<
/Linearized 1
/O 1166
/H [ 1410 370 ]
/L 259280
/E 78778
/N 13
/T 235900
>>
endobj
xref
1163 22
0000000016 00000 n
Test your knowledge with gamified quizzes. Separation of chlorophyll and carotenoid pigments are done using paper chromatography. Pigments appear the color of the reflected light, so the chlorophyll pigments do not use the green portion of the spectrum.  <>/XObject<>>>/Type/XObject/Subtype/Form/BBox[0 0 595 842]/Matrix[1 0 0 1 0 0]/FormType 1>>stream C) Chromatographic separation of pigments by column chromatography (CC). Over 10 million students from across the world are already learning smarter. Lab 4B proves that light and chloroplasts are required for the light reactions of photosynthesis to occur. Chlorophyll c has a very low value somewhere around 0.1 or less in a solvent of In other
<>/XObject<>>>/Type/XObject/Subtype/Form/BBox[0 0 595 842]/Matrix[1 0 0 1 0 0]/FormType 1>>stream C) Chromatographic separation of pigments by column chromatography (CC). Over 10 million students from across the world are already learning smarter. Lab 4B proves that light and chloroplasts are required for the light reactions of photosynthesis to occur. Chlorophyll c has a very low value somewhere around 0.1 or less in a solvent of In other
For example, plants have two types of chlorophyll molecules, chlorophyll a & b. $$Rf=\dfrac{\text{Distance travelled by compound}}{\text{Distance travelled by solvent}}$$. Can chlorophyll be separated by chromatography? WebBacteriochlorophyll c has an RF of about 0.7; this is based on a petroleum solvent. What is the best solvent for leaf chromatography? endstream endobj startxref WebThe pigments move up the paper with the chromatography solvent, BUT not at the same rate. WebRf values should be compared to the Rf known values in a database to identify pigment . 11 0 obj Repeat this process until you have added five additional drops of solution, allowing each to dry before applying the next. These pigments mainly absorb purple light, which has more energy. <>/Border[0 0 0]/P 3 0 R>>
 However, a pure compound will show only a single spot - no matter the solvent used. Under a hood, add 1 cm of chromatography solvent to your test tube and place this in a test tube rack (label your tube if multiple people are using the same rack). In other words, what chlorophyll chromatography solvents are used to help create this phase? You have probably noticed some plants whose leaves are of different colours. When looking at the databases, ensure that they are for paper chromatography and use the same solvent as these variables will make results differ. Tube"L" (mg Chl / mL): 0.0533 x Absorbance (0.30) = 0.01599mg Chl / mL Remember that your enriched, diluted chloroplast suspension (Tube"L") was created by diluting 100 L ofenriched, undiluted suspension (Tube"E") into 3.5 mL phosphate buffer. '7h8Udcz4&[Xd3k=RR ]j`mH]ZUI,^'%=)LE
i^n%BNIdveEJR+zBGA1@CoW!_kG~ d3SB?#?
However, a pure compound will show only a single spot - no matter the solvent used. Under a hood, add 1 cm of chromatography solvent to your test tube and place this in a test tube rack (label your tube if multiple people are using the same rack). In other words, what chlorophyll chromatography solvents are used to help create this phase? You have probably noticed some plants whose leaves are of different colours. When looking at the databases, ensure that they are for paper chromatography and use the same solvent as these variables will make results differ. Tube"L" (mg Chl / mL): 0.0533 x Absorbance (0.30) = 0.01599mg Chl / mL Remember that your enriched, diluted chloroplast suspension (Tube"L") was created by diluting 100 L ofenriched, undiluted suspension (Tube"E") into 3.5 mL phosphate buffer. '7h8Udcz4&[Xd3k=RR ]j`mH]ZUI,^'%=)LE
i^n%BNIdveEJR+zBGA1@CoW!_kG~ d3SB?#?  The pigments were identified by comparing the Rf values to the known Rf values of these pigments. HUMo8b_"E ]`[=bDCbRN,!7f#%DOVd]^2%[T4fhZ4YYYw#iqCeR]\[53Dp^x2DyQS!C*:B?l=[ y$* mx9C>me{$E
~K1=]Qg9YQM:yZ What does Dunknown signify? endobj trailer
<<
/Size 1185
/Info 1148 0 R
/Root 1164 0 R
/Prev 235888
/ID[<43386042eae72abdf67d0671fa8f2d1b>]
>>
startxref
0
%%EOF
1164 0 obj
<<
/Type /Catalog
/Pages 1151 0 R
/Metadata 1149 0 R
/OpenAction [ 1166 0 R /XYZ null null null ]
/PageMode /UseNone
/JT 1162 0 R
/PageLabels 1147 0 R
/StructTreeRoot 1165 0 R
/PieceInfo << /MarkedPDF << /LastModified (D:20020609213337)>> >>
/LastModified (D:20020609213337)
/MarkInfo << /Marked true /LetterspaceFlags 0 >>
>>
endobj
1165 0 obj
<<
/Type /StructTreeRoot
/RoleMap 43 0 R
/ClassMap 46 0 R
/K [ 786 0 R 787 0 R 788 0 R 789 0 R 790 0 R 791 0 R 792 0 R 793 0 R 794 0 R
795 0 R 796 0 R 797 0 R 798 0 R 799 0 R 800 0 R ]
/ParentTree 1077 0 R
/ParentTreeNextKey 13
>>
endobj
1183 0 obj
<< /S 320 /L 389 /C 405 /Filter /FlateDecode /Length 1184 0 R >>
stream
Yes, chlorophyll pigments can be separated by paper chromatography based on their solubility and size. NF2i~S&fJKx3) YjbV)xrlvWh1_UQ($>N)g8u: 0000003283 00000 n
oGNjN7jzLssa.h]L5'f@RJ Latest answer posted July 17, 2012 at 2:55:17 PM. Rf value can be indicative of a substance's solubility in the solvent and/or size. endobj Chromatography is a Greek phrase that combines the terms "chromo" and "graph", which together mean "colour writing". Log in here. Webisolate and study the photosynthetic pigments, chlorophyll a, chlorophyll b, and carotenoids. When the solvent has traveled up the TLC strip about 1 cm from the top of the strip, remove the strip from the test tube and draw a line in pencil at the edge of the solvent front. Which pigments were the most nonpolar (least polar, highest Rf values)? The "loading line" is the location of the original pigment line painted on the paper. WebThe Retention factor or Rf value applies to chromatography to make the technique scientific. How does chromatography identify chlorophyll?
The pigments were identified by comparing the Rf values to the known Rf values of these pigments. HUMo8b_"E ]`[=bDCbRN,!7f#%DOVd]^2%[T4fhZ4YYYw#iqCeR]\[53Dp^x2DyQS!C*:B?l=[ y$* mx9C>me{$E
~K1=]Qg9YQM:yZ What does Dunknown signify? endobj trailer
<<
/Size 1185
/Info 1148 0 R
/Root 1164 0 R
/Prev 235888
/ID[<43386042eae72abdf67d0671fa8f2d1b>]
>>
startxref
0
%%EOF
1164 0 obj
<<
/Type /Catalog
/Pages 1151 0 R
/Metadata 1149 0 R
/OpenAction [ 1166 0 R /XYZ null null null ]
/PageMode /UseNone
/JT 1162 0 R
/PageLabels 1147 0 R
/StructTreeRoot 1165 0 R
/PieceInfo << /MarkedPDF << /LastModified (D:20020609213337)>> >>
/LastModified (D:20020609213337)
/MarkInfo << /Marked true /LetterspaceFlags 0 >>
>>
endobj
1165 0 obj
<<
/Type /StructTreeRoot
/RoleMap 43 0 R
/ClassMap 46 0 R
/K [ 786 0 R 787 0 R 788 0 R 789 0 R 790 0 R 791 0 R 792 0 R 793 0 R 794 0 R
795 0 R 796 0 R 797 0 R 798 0 R 799 0 R 800 0 R ]
/ParentTree 1077 0 R
/ParentTreeNextKey 13
>>
endobj
1183 0 obj
<< /S 320 /L 389 /C 405 /Filter /FlateDecode /Length 1184 0 R >>
stream
Yes, chlorophyll pigments can be separated by paper chromatography based on their solubility and size. NF2i~S&fJKx3) YjbV)xrlvWh1_UQ($>N)g8u: 0000003283 00000 n
oGNjN7jzLssa.h]L5'f@RJ Latest answer posted July 17, 2012 at 2:55:17 PM. Rf value can be indicative of a substance's solubility in the solvent and/or size. endobj Chromatography is a Greek phrase that combines the terms "chromo" and "graph", which together mean "colour writing". Log in here. Webisolate and study the photosynthetic pigments, chlorophyll a, chlorophyll b, and carotenoids. When the solvent has traveled up the TLC strip about 1 cm from the top of the strip, remove the strip from the test tube and draw a line in pencil at the edge of the solvent front. Which pigments were the most nonpolar (least polar, highest Rf values)? The "loading line" is the location of the original pigment line painted on the paper. WebThe Retention factor or Rf value applies to chromatography to make the technique scientific. How does chromatography identify chlorophyll?
%PDF-1.3
%
For example, salt, being soluble in water but insoluble in oil, would have a different RF value for each. Pigments with small Rf values are either less soluble in the solvent, large in size and/or have a greater affinity for the stationary phase (paper) than those with larger Rf values.  If there were polar pigments in the leaves and you used a nonpolar solvent to extract the pigments from the leaf, would they dissolve and be present in the solution you used to run your TLC experiment? Photosynthetic pigments found in chloroplasts can be classified into two main groups based on the colours of the light they absorb, The retention factor (Rf) is used in paper chromatography to compare and identify the separated chemical substances.$$Rf=\dfrac{\text{Distance travelled by compound}}{\text{Distance travelled by solvent}}$$. 9 0 obj Pheophytin Grey 0 0. These pigments include two greenish Their cells contain structures that capture energy from the sun. SV;*P vnM}kdQ[d&Mer^f0x^6k2[eviTfUCgxUgudy!o&r1#vwv6sB It is especially useful for separating complex mixtures of amino acids, carbohydrates, lipids, and nucleic acids, which are often difficult to separate using other methods. endobj In the experiment pictured at left, the solvent used was comprised of nine parts petroleum ether and one part acetone. The distance travelled by pigment chlorophyll a was 14.5cm with a Rf value of 1.04 and chlorophyll b reached 14cm with a Rf value of 1.00. Prepare your TLC strip by drawing a line across the paper in pencil 2 cm from the bottom of the strip and set aside. This is a 36-fold dilution. Our summaries and analyses are written by experts, and your questions are answered by real teachers. Lutein Yellow 0- 0. "What is the RF value of chlorophyll c, chlorophyll d, and bacteriocholrophyll c?" 0000004064 00000 n
The word comes from chromatography when it was discovered that 0000004033 00000 n
Next, measure the distance from where the pigment started to the farthest point that each pigment traveled. <>/Border[0 0 0]/P 3 0 R>> endstream
endobj
224 0 obj
<>stream
High Rf values from TLC using a nonpolar solvent means the pigment is more nonpolar. Draw or tape your TLC strip and label as many pigments as you can (see the next page for more information on pigments). 0
Have you ever wondered why that is? For yellow, Rf=0.89; for orange, Rf=0.78; for dark green, Rf=0.51; for green, Rf=0.49; and for light green, Rf=0.44. %PDF-1.5
%
Pigments are separated according to differences in their relative solubilities.
If there were polar pigments in the leaves and you used a nonpolar solvent to extract the pigments from the leaf, would they dissolve and be present in the solution you used to run your TLC experiment? Photosynthetic pigments found in chloroplasts can be classified into two main groups based on the colours of the light they absorb, The retention factor (Rf) is used in paper chromatography to compare and identify the separated chemical substances.$$Rf=\dfrac{\text{Distance travelled by compound}}{\text{Distance travelled by solvent}}$$. 9 0 obj Pheophytin Grey 0 0. These pigments include two greenish Their cells contain structures that capture energy from the sun. SV;*P vnM}kdQ[d&Mer^f0x^6k2[eviTfUCgxUgudy!o&r1#vwv6sB It is especially useful for separating complex mixtures of amino acids, carbohydrates, lipids, and nucleic acids, which are often difficult to separate using other methods. endobj In the experiment pictured at left, the solvent used was comprised of nine parts petroleum ether and one part acetone. The distance travelled by pigment chlorophyll a was 14.5cm with a Rf value of 1.04 and chlorophyll b reached 14cm with a Rf value of 1.00. Prepare your TLC strip by drawing a line across the paper in pencil 2 cm from the bottom of the strip and set aside. This is a 36-fold dilution. Our summaries and analyses are written by experts, and your questions are answered by real teachers. Lutein Yellow 0- 0. "What is the RF value of chlorophyll c, chlorophyll d, and bacteriocholrophyll c?" 0000004064 00000 n
The word comes from chromatography when it was discovered that 0000004033 00000 n
Next, measure the distance from where the pigment started to the farthest point that each pigment traveled. <>/Border[0 0 0]/P 3 0 R>> endstream
endobj
224 0 obj
<>stream
High Rf values from TLC using a nonpolar solvent means the pigment is more nonpolar. Draw or tape your TLC strip and label as many pigments as you can (see the next page for more information on pigments). 0
Have you ever wondered why that is? For yellow, Rf=0.89; for orange, Rf=0.78; for dark green, Rf=0.51; for green, Rf=0.49; and for light green, Rf=0.44. %PDF-1.5
%
Pigments are separated according to differences in their relative solubilities.