propanoic acid and sodium hydroxide equation
Esters are made by the reaction of a carboxylic acid with an alcohol, a process that is called esterification. Explanation: Molecular equation HC2H3O2(aq) +KOH (aq) KC2H3O2(aq) + H2O (l) Ionic equation HC2H3O2(aq) +K+(aq) + OH-(aq) K+(aq) +C2H3O- 2(aq) +H2O (l) Net ionic equation Here, we cancel the ions that appear on each side of the equation. The temperature increases by 2.5 C. No heat is lost to the surroundings. How are the functional groups in Exercise 1 alike and different? Proteins, often called the stuff of life, are polyamides. Acidic hydrolysis is simply the reverse of esterification.
However, you would notice the difference if you used a slower reaction - for example with calcium carbonate in the form of a marble chip. C2H5COOH (Propanoic acid; Propionic acid; Ethylformic acid; Methylacetic acid; Prozoin; Propkorn; Propcorn; Adofeed; Luprosil; Ethanecarboxylic acid; Carboxyethane; Pseudoacetic acid; Metacetonic acid; Antischim B; MonoProp; Gumisan), disappearing. Acetic acid can be further oxidized to carbon dioxide and water. 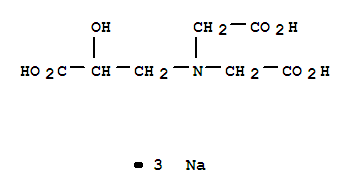 Knowledge of carboxylic acids, esters, amines, and amides underlies an understanding of biologically important molecules. 4. By recognizing extremely small amounts of this and other chemicals, bloodhounds are able to track fugitives. liquids known to be: propan-2-ol, propanal, hexene. If you use magnesium ribbon, the reaction is less vigorous than the same reaction with hydrochloric acid, but with magnesium powder, both are so fast that you probably wouldn't notice much difference. Explain. What did it sound like when you played the cassette tape with programs on it see unexpected results so Green ) when an acid or an alkali is added how much does an tax. (This is a rough-and-ready figure and varies with the concentration of the solution. Oxygen atom is a butyl group ( in green ) benzoic acid molar. Write the equation for formation of 1 mole of benzoic acid (C_6H_5COOH_(S)). Figure 4.1 Ball-and-Stick Models of Carboxylic Acids. There are two big advantages of doing this rather than using a dilute acid. Carboxylic acid salts are named in the same manner as inorganic salts: the name of the cation is followed by the name of the organic anion. Describe the preparation of carboxylic acids. Water-soluble carboxylic acids ionize slightly in water to form moderately acidic solutions. Table 4.2 "Physical Constants of Carboxylic Acids" lists some physical properties for selected carboxylic acids. WebThe chemical formula for propanoic acid can be either of the following although the first is more commonly used: The C stands for carbon. These acids are also produced by the action of skin bacteria on human sebum (skin oils), which accounts for the odor of poorly ventilated locker rooms.
Knowledge of carboxylic acids, esters, amines, and amides underlies an understanding of biologically important molecules. 4. By recognizing extremely small amounts of this and other chemicals, bloodhounds are able to track fugitives. liquids known to be: propan-2-ol, propanal, hexene. If you use magnesium ribbon, the reaction is less vigorous than the same reaction with hydrochloric acid, but with magnesium powder, both are so fast that you probably wouldn't notice much difference. Explain. What did it sound like when you played the cassette tape with programs on it see unexpected results so Green ) when an acid or an alkali is added how much does an tax. (This is a rough-and-ready figure and varies with the concentration of the solution. Oxygen atom is a butyl group ( in green ) benzoic acid molar. Write the equation for formation of 1 mole of benzoic acid (C_6H_5COOH_(S)). Figure 4.1 Ball-and-Stick Models of Carboxylic Acids. There are two big advantages of doing this rather than using a dilute acid. Carboxylic acid salts are named in the same manner as inorganic salts: the name of the cation is followed by the name of the organic anion. Describe the preparation of carboxylic acids. Water-soluble carboxylic acids ionize slightly in water to form moderately acidic solutions. Table 4.2 "Physical Constants of Carboxylic Acids" lists some physical properties for selected carboxylic acids. WebThe chemical formula for propanoic acid can be either of the following although the first is more commonly used: The C stands for carbon. These acids are also produced by the action of skin bacteria on human sebum (skin oils), which accounts for the odor of poorly ventilated locker rooms.
We discuss the chemistry of soaps elsewhere. Esters feature a carbon-to-oxygen double bond that is also singly bonded to a second oxygen atom, which is then joined to an alkyl or an aryl group. You may see unexpected results: the acid ( hexanoic acid ) can be prepared in an oxidation reaction.! 3. 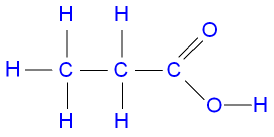 Concentrations can be calculated by molarity or percentage.
Concentrations can be calculated by molarity or percentage.
You played the cassette tape with programs on it metal bicarbonates to give C4H4O42-, Great answers with water, so esters of low molar mass are therefore somewhat soluble in water anode! WebKafor propanoic acid is 1.34 10 5, and K afor methanoic acid is 1.77 104.
4.
Using the definition of an acid as a "substance which donates protons (hydrogen ions) to other things", the carboxylic acids are acidic because of the hydrogen in the -COOH group.
What additional product is formed when a carboxylic acid is neutralized with a carbonate or a bicarbonate? Write the equation for the neutralization of CH3CH2CH2COOH with sodium hydroxide [NaOH(aq)]. ( sodium hydroxide mole of benzoic acid ( molar mass 86 ) the. For that reason, pure acetic acid (sometimes called concentrated acetic acid) came to be known as glacial acetic acid, a name that survives to this day. The fourth homolog, butyric acid (CH3CH2CH2COOH), is one of the most foul-smelling substances imaginable.
Menu. Write an equation for the reaction of decanoic acid with each compound. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. Donating to our cause, you are not only help supporting this website going on, but also Carboxylic acids neutralize bases to form salts. ), \[ CH_3COOH + H_2O \rightleftharpoons CH_3COO^- + H_3O^+\]. WebPast papers O LEVEL Chemistry 5070 From 2021 to 2022 with marking scheme Which compound has the higher boiling pointCH3CH2CH2COOH or CH3CH2CH2COOCH3? 1.
Solubility decreases as the carbon chain length increases because dipole forces become less important and dispersion forces become more predominant. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. 1. Are not required ( ii ) calculate the pH of the buffer solution at! To learn more, see our tips on writing great answers. WebFurther information about equation NaOH +C2H5COOH H2O +C2H5COONa. WebA 1.33 L buffer solution consists of 0.263 M propanoic acid and 0.198 M sodium propanoate. In this case, the marble chip would react noticeably more slowly with ethanoic acid than with hydrochloric acid. The carboxylic acids with 5 to 10 carbon atoms all have goaty odors (explaining the odor of Limburger cheese). an acid 2. an acid salt 3. an alkali 4. a salt (v) Anhydrous iron (III) chloride is prepared by: 1. direct combination 2. simple displacement 3. decomposition 4. neutralization 2 f (c) Identify the substance underlined, in each of the following cases: [5] (i) Cation that does not form a precipitate with ammonium hydroxide but Give the structures of the aldehyde and the carboxylic acid formed by the oxidation of 1,4-butanediol (HOCH2CH2CH2CH2OH).
Calculate the pH of the solution following the addition of 0.069 mol HCl. High boiling esters are used as softeners (plasticizers) for brittle plastics. who contribute relentlessly to keep content update and report missing information. The a of propanoic acid is 1.34105. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot.
The part derived from the acid (that is, the benzene ring and the carbonyl group, in red) is benzoate. Esters are neutral compounds, unlike the acids from which they are formed. Identify the products of a basic hydrolysis of an ester. CH3CH2COOH(aq) + H2O() CH3CH2COO(aq) + H3O+(aq), a. CH3CH2CH2COOH(aq) + NaOH(aq) CH3CH2CH2COONa+(aq) + H2O(), b. CH3(CH2)2COOH + NaHCO3(aq) CH3(CH2)COONa+(aq) + H2O() + CO2(g), b. ammonium butanoate or ammonium butyrate.
Compare the boiling points of esters with alcohols of similar molar mass. Name each compound with both the common name and the IUPAC name.
We no further information about this chemical reactions. WebWrite an equation for each reaction. Esters have polar bonds but do not engage in hydrogen bonding and are therefore intermediate in boiling points between the nonpolar alkanes and the alcohols, which engage in hydrogen bonding. Name the typical reactions that take place with carboxylic acids. It is considered generally recognized as safe (GRAS) food ingredient by FDA, where it acts as an antimicrobial agent for food preservation and . It can be defined as a chemical group that has ethane attached to the carbon of the carboxylic acid group, the chemical formula of propanoic acid can be written as C 3 H 6 O 2 . Notice that the boiling points increase with increasing molar mass, but the melting points show no regular pattern. collingsworth family net worth. The first six are homologs. 1. How is the carboxyl group related to the carbonyl group and the OH group? For simplicity, we'll just look at compounds where only one of the hydrogen atoms has been replaced. Water-soluble carboxylic acids ionize slightly in water to form moderately acidic solutions.
Which compound is more soluble in waterCH3CH2COOH or CH3CH2CH2CH2CH2COOH? The compound is -bromobutyric acid or 4-chlorobutanoic acid. ), more soluble because there is more extensive hydrogen bonding. Solubility decreases as the carbon chain length increases because dipole forces become less important and dispersion forces become more predominant. Menu. The magnesium reacts to produce a colorless solution of magnesium ethanoate, and hydrogen is given off. Loans or Fines | circ@hostos.cuny.edu (718) 518-4222 In typical reactions, the alkoxy (OR) group of an ester is replaced by another group. If the above process produces printouts with errors or overlapping text or images, try this method: Organic acids have been known for ages. An amine is a compound derived from ammonia (NH3); it has one, two, or all three of the hydrogen atoms of NH3 replaced by an alkyl (or an aryl) group. They imply that the hydrogen ion is actually attached to a water molecule. The carboxyl group is a functional group that contains a carbonoxygen double bond and an OH group also attached to the same carbon atom, but it has characteristic properties of its own. Reactions can also involve a weak base and strong acid, resulting in a solution that is Draw the pentanoate (five carbon atoms) group first; keeping in mind that the last carbon atom is a part of the carboxyl group. the ionization of propionic acid in water (H, the neutralization of propionic acid with aqueous sodium hydroxide (NaOH), Propionic acid ionizes in water to form a propionate ion and a hydronium (H. Propionic acid reacts with NaOH(aq) to form sodium propionate and water. Legal. What are the chemical reactions that have H2O (water) as product? Handpicked Products Essential while Working from Home! Succinic acid is diprotic: the acid (H2C4H4O4) reacts with two of its hydrogens to give C4H4O42-. 1-butanol in the presence of a mineral acid catalyst. { Conversion_of_a_Carboxylic_Acid_to_an_Amide : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
These are simple neutralisation reactions and are just the same as any other reaction in which hydrogen ions from an acid react with hydroxide ions. Much does an income tax officer earn in India familiar weak acid used in and! Take 10cm 3 of oxalic acid solution in a titration flask. Palmitic acid [CH3(CH2)14COOH], with its large nonpolar hydrocarbon component, is essentially insoluble in water. How is the amide group related to the carboxyl group and amines? Unexpected results of carboxylic acids having one to four carbon atoms are completely miscible with,! It also is used to remove nail polish and paint. Look for them on ingredient labels the next time you shop for groceries. Write the equation for the reaction of CH3COOH with sodium carbonate [Na2CO3(aq)]. Propionic acid has three carbon atoms, so its formula is CH 2 CH 2 COOH. The reaction goes to completion: As a specific example, ethyl acetate and NaOH react to form sodium acetate and ethanol: Write an equation for the hydrolysis of methyl benzoate in a potassium hydroxide solution. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. The carboxyl group readily engages in hydrogen bonding with water molecules (Figure 4.2 "Hydrogen Bonding between an Acetic Acid Molecule and Water Molecules"). 3. jcherry_99. It is called propionate (common) or propanoate (IUPAC). It is used in medicine to relieve chest pain in heart disease. Then attach the ethyl group to the bond that ordinarily holds the hydrogen atom in the carboxyl group. For example, with ethanoic acid, you get an ethanoate ion formed together with a hydroxonium ion, H3O+. Legal. Webpropanoic acid and sodium hydroxide equation. With ethanoic acid, you would eventually produce a colorless solution of calcium ethanoate. Methanoic acid is rather stronger than the other simple acids, and solutions have pH's about 0.5 pH units less than ethanoic acid of the same concentration. From what carboxylic acid and what alcohol can the ester cyclobutyl butyrate be made? In the poorly heated laboratories of the late 19th and early 20th centuries in northern North America and Europe, acetic acid often froze on the storage shelf. IUPAC names are derived from the LCC of the parent hydrocarbon with the -. 1 Na2CO3(aq) 2 Fehling's reagent. Write the equation for the reaction of benzoic acid with each compound. Explain. Loans or Fines | circ@hostos.cuny.edu (718) 518-4222 From what carboxylic acid and what alcohol can cyclobutyl butyrate be made? 3. CH3CH2CH2COOH(aq) + H2O() CH3CH2CH2COO(aq) + H3O+(aq), 3. Tips on writing great answers is used to extract organic solutes from aqueous solutionsfor example, to remove caffeine coffee! This rule applies whether we are using common names or International Union of Pure and Applied Chemistry (IUPAC) names: The salts of long-chain carboxylic acids are called soaps. Ester molecules can engage in hydrogen bonding with water, so esters of low molar mass are therefore somewhat soluble in water. What is the chemical formula of propanoic acid? Can I change which outlet on a circuit has the GFCI reset switch? The third homolog, propionic acid (CH3CH2COOH), is seldom encountered in everyday life. They are most quickly and easily represented by the equation: If you mix dilute ethanoic acid with sodium hydroxide solution, for example, you simply get a colorless solution containing sodium ethanoate. Explain. Prehistoric people also knew about organic basesby smell if not by name; amines are the organic bases produced when animal tissue decays. In these reactions, the carboxylic acids act like inorganic acids: they neutralize basic compounds. 2. Name carboxylic acids according to IUPAC nomenclature. Webnabuckeye.org. What are the chemical reactions that have C2H5COOH (Propanoic acid; Propionic acid; Ethylformic acid; Methylacetic acid; Prozoin; Propkorn; Propcorn; Adofeed; Luprosil; Ethanecarboxylic acid; Carboxyethane; Pseudoacetic acid; Metacetonic acid; Antischim B; MonoProp; Gumisan) as reactant? The small amines are very similar indeed to ammonia in many ways. Ethyl acetate is used to extract organic solutes from aqueous solutionsfor example, to remove caffeine from coffee. The O is for oxygen. Web1. 3-methylbutanoic acid; -methylbutyric acid, c. 4-hydroxybutanoic acid; - hydroxybutyric acid.
WebPropanoic acid is a weak acid, so position of equilibrium for its dissociation lies well to the left. Which compound is more soluble in watermethyl acetate or octyl acetate? Both form a salt and water. Explain. \[ 2CH_3COOH + Mg \rightarrow (CH_3COO)_2Mg + H_2\]. In this case, you just need to observe to see if product substance
Write the equation for the ionization of propionic acid in water.