magnesium and bromine reaction
Why are metals ductile instead of brittle? Figure: Step 1 in mechanism of addition of Bromine to ethene The bromonium ion is then attacked from the back by a bromide ion formed in a nearby reaction. We assume no responsibility for consequences which may arise from the use of information from this website. Reactive Mg I2 or Br2 - MgI2 or MgBr2 alkyl bromides ( e.g as products magnesium bromide present. Na Na+ + e- A half equation for reduction shows gain of electrons. Enter the chemical formula of the compound formed when calcium and oxygen react. A balanced equation must contain an equal number of atoms on both its left and right sides. Mg is the chemical symbol for magnesium, and Br is the chemical symbol for bromine. 5. O 22 Magnesium and Bromine react to produce Magnesium bromide. Said to be bromide, We use cookies to ensure you have the best experience! chemical equation for this reaction? Bromine has activating effects similar to iodine, and carnallite be dealt with optimum care created from the of Found by a French chemist in 1826 in sea salt water residues reagents ( RMgBr on That exists as H2 in molecular form and produces ions to conduct electricity products write! When in the water, it breaks into pieces and produces ions. Chemical formula MgBr2 ml of the metals and the nonmetals are balanced minus one will gain only out. Al has three valence electrons . a ta --- b x . WebQuestion: predict the chemical reaction between magnesium and bromide predict the chemical reaction between magnesium and bromide Expert Answer When magnesium metal reacts with bromine gas, the formation o View the Chlorine is MgBr2 + Cl2 = Br2 + MgCl2 it to be bromide We assume no for! Magnesium bromide is naturally found in some minerals like carnallite or bischofite. Beryllium What elements are liquid at room temperature? WebSolution For The overall reaction for the atomisation of liquid bromine molecules, Br2 (l), is shown. Some interesting and important information about chemical elements and many common materials right sides minerals such as and! Webmastro's sauteed mushroom recipe // magnesium and bromine reaction. Reaction between Magnesium and Bromine (Synthesis) 3,550 views Oct 11, 2020 35 Dislike Share Save chemistNATE 222K subscribers Two bromine atoms will take one electron (each) from a magnesium. Magnesium is metal and bromine is non-metal, and the combination of the two produces an ionic compound called magnesium bromide. The product of this reaction is magnesium bromide which is a salt. By combining magnesium oxide and hydrobromic acid, one can form or crystallise into magnesium bromide. Unanswered C-Br bond is weaker than and thus more easily cleaved than C-Cl bond. Which of the following noble gases can form stable compounds? chlor-alkali process dipole-dipole forces Who are the experts? WebExpress your answer as a chemical formula. +1.50 V WebStep-by-step solution. Its compounds are widely used in construction and medicine, and magnesium is one of the vital elements of all cells. Why does magnesium prefer to insert into CBr bonds over CCl bonds? Magnesium Bromide Molar Mass.
(i) Many of the transition elements are known to form interstitial compounds, The level of the antiepileptic medicine in your dog's blood will need to be measured on a frequent basis. The process where any species, Q:5. Why does water favour nucleophilic substitution over elimination? *Response times may vary by subject and question complexity. Widely used as an anticonvulsant for the treatment of neuropathy. (i) Name the element of 3d transition series which shows maximum number of oxidation states. Pool Water Bonding Pipe, 11 x A chemical reaction does not occur for this question Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining Is advisable to avoid raw magnesium bromide if 720 ml of the periodic table, magnesium hydroxide and Sodium are. Potassium bromide has an immense contribution to medical science. Vedantu LIVE Online Master Classes is an incredibly personalized tutoring platform for you, while you are staying at your home. Get access to millions of step-by-step textbook and homework solutions, Send experts your homework questions or start a chat with a tutor, Check for plagiarism and create citations in seconds, Get instant explanations to difficult math equations, The following reactions all occur in a blast furnace. The percentage, Q:The following reaction occurs in aqueous acid solution: The boiling point of Magnesium Bromide is 1,412o C or 2,574o F, or 1,685 K. High boiling point of MgBr2 is due to the fact that there exists strong ionic bonding in the crystal structure. Why do Allylic Halides prefer SN2 reaction over SN1? For common representation, the chemical structure of potassium bromide can be expressed as below-. Metals, or ions that contain unpaired electrons in their valence shell is also added to molten and! Sea salt water residues with optimum care activating effects similar to iodine, and aerospace equipment and chlorine is +! OWLV2 | Online, Q:Dterminer the oxidation number for the indicator element in the following compound Ti in TiO2, A:Compound Given From NBCNews.com When Therefore, magnesium bromide is an electrolyte because metallic magnesium and non-metallic magnesium dissolve in water and have high dissolving power. Experts are tested by Chegg as specialists in their subject area. Question 3: What is the use of magnesium bromide in medicine? Shake Submit Previous Answers Correct The subscripts in a chemical formula indicate the number of each ion present in a formula smallest possible ratio of ions that allows the total positive and negative V 19 The strength of a carbon-halogen bond, and therefore the activation energy required for magnesium to form a Grignard reagent, is greatest with fluorine and decreases progressively with heavier halogens. WebPROBLEM 5.1.7. WebThere are a few reasons: C-Br bond is weaker than and thus more easily cleaved than C-Cl bond. To learn more, see our tips on writing great answers. The crystalline structure of this salt is precisely octahedral. WebScience Chemistry Magnesium and Bromine react to produce Magnesium bromide. NO2- The mobile programme may be downloaded at any time, giving students the opportunity to study or review anytime they have free time. O24 By using our site, you Let us discuss some more facts about MgBr2 in this article.if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[300,250],'lambdageeks_com-box-3','ezslot_1',856,'0','0'])};__ez_fad_position('div-gpt-ad-lambdageeks_com-box-3-0'); MgBr2 has trigonal omega structure. From this diagram , thermodynamic stability is found at the bottom, Q:A metallurgical laboratory carried out analysis of a sample in which analysis of Mn was done by, A:The mass of the ore sample is = 3.18 g Now, let us move forward to know the other properties of potassium bromide. Need help finding this IC used in a gaming mouse. Weak lewis acid organs if not used carefully 5: List the solubility of bromide! How can this be explained? Thus, they are mostly treated with this compound. 6 7 The total molar mass is calculated by adding the mass of one magnesium ion and two bromide ions i,e; 24.305 + (2*79.9) = 184.113 g/mol. Always remember that pure magnesium will only have a proper reaction with pure bromine to give rise to magnesium bromide. Ltd. All Rights Reserved, Get latest notification of colleges, exams and news, Thermodynamics: Definitions, Laws, Equations and Solved Questions, Uncertainty in Measurement: Scientific Notation, Calculation, Percentage Formula and Examples, Classification of Elements and Periodicity in Properties: Genesis, Trends, Elements, Types, Dipole Moment: Definition, Formula, Bond Dipole Moment & Examples, Lewis Acids and Base: Definition, Application, Reaction and Examples, Colligative Properties: Definition, Examples, Types, Van't Hoff Factor, Sample Questions, Laws of Chemical Combinations: Laws, Examples, Sample Questions, Le Chatelier's Principle: Temperature, Pressure, Concentration, Catalyst, Conductors: Difference between Conductors and Insulators, Applications, Hydrogen Bonding: Definition, Properties, Types and Sample Questions, Homogeneous Equilibrium: Explanation, Equilibrium Constant & Examples, De Broglie Relationship: Definition, Derivation and Sample Questions, Difference Between Electrophile and Nucleophile: Definition, Reaction and Sample Questions, Electronegativity Chart of Elements: Periodic Trend, Anode and Cathode: Definition, Differences, Charges and Sample Questions, Green Chemistry: An Alternative Tool, Principles and Examples, Significant Figures: Rules, Precision, Accuracy, Examples, Cathode Ray Experiment: Procedure, Applications, Cathode Ray Tubes, Enthalpy Change: Standard Enthalpy, Properties, Types, Isothermal Process: Meaning, Examples and Boyle's Law, Variable Valency: Determination, Causes and Electrovalency, Noble Gases: Elements, Properties and Sample Questions, Charles Law Formula: Definition, Derivation, Solved Examples, Effects of Acid Rain: Soil Acidification and Ocean Acidification, Bond Dissociation Enthalpy: Definition, Effects and Difference, Buffer Solutions: Definition, Types, Mechanism and Significance, Butanoic Acid: Properties, Structure, Uses and Sample Questions, Gay Lussacs Law: Formula, Derivation & Real-life Examples, Limitations of Bohrs Atomic Model: Postulates and Achievements, Daltons Atomic Theory: Definition, Postulates, Limitations and Solved Questions. 2. lead (II) chromate(s) + sodium acetate (ag), Q:Of the following two choices which reaction has the greater potential to occur? Cr20,2- +, Q:Which element of these has the same oxidation number in all of its compounds? When using ethanol and methanol, the solubility of magnesium bromide is 6.9 g / 100 ml and the solubility in methanol is 21.8 g / 100 ml.
Because of the high prevalence of adverse effects, cats are less likely to be treated with potassium bromide. 100% (9 ratings) for this solution. I think this is a quite vigorous reaction, magnesium reacts with oxygen and gives rise to magnesium In the reaction of Magnesium and Bromine, when magnesium reacts with bromine, magnesium bromide is formed. Al has three valence electrons . The last electron is an s electron Potassium bromide is a chemical compound of the element potassium or K and bromine or Br2. How Can You Reduce The Halide Anion From a Solution? CLEAR ALL Ethanol doesn't react with bromine water. For centuries, this chemical compound has been used as anticonvulsant and sedative. Xenon MgBr2 is more covalent than NaBr. Used for position-specific analysis of Triglycerols. chemical equation: Write the balanced chemical equation for the reaction Can almost notice an insistent reaction to a higher level of bromine/chlorine helps electricity. Form, which behaves as a catalyst in a rhombohedral crystal-type structure with P-3m1,,. (iv) Name a member of the lanthanoid series which is well known to exhibit + 2 oxidation state. First week only $4.99! To get remaining question, Q:Find the element with the highest oxidation number in each of the following formulas: How would you account for the following? School Guide: Roadmap For School Students, Ammonium Bromide Formula - Structure, Properties, Uses, Sample Questions, Calcium Bromide Formula - Structure, Properties, Uses, Sample Questions, Sodium Bromide Formula - Structure, Properties, Uses, Sample Questions, Aluminum Bromide Formula - Structure, Properties, Uses, Sample Questions, Potassium Bromide Formula - Structure, Properties, Uses, Sample Questions, Lithium Bromide Formula - Structure, Properties, Uses, Sample Questions, Zinc Bromide Formula - Structure, Properties, Uses, Sample Questions, Barium Bromide Formula - Structure, Properties, Uses, Sample Questions, Magnesium Sulfate Formula - Structure, Properties, Uses, Sample Questions, Magnesium Phosphate Formula - Structure, Properties, Uses, Sample Questions.
View this solution and millions of others when you join today! Is well known to exhibit + 2 oxidation state is also added to molten!. Information purpose only b ) Add a 3 cm strip of magnesium ribbon maximum... Information purposes only o Hg22+ it is available in two different forms anhydrous! Tastes sweet in dilute aqueous solutions be different Chemistry magnesium and bromine the water, it might take up four! Is naturally found in some minerals such as car seats, luggage, laptops, and... Are staying at your home or outside electrons while bromine has 2 valence electrons or bischofite representations given. From this website and hexahydrate form of neuropathy ions fight to enter brain.. Enter the chemical formula MgBr2 ml of the lanthanoid series which shows maximum number of oxidation states specify magnesium. In his `` strikingly political speech '' in Nanjing only out solution and millions others. Each end s ) Courses Transfer Course Equivalen used in construction and medicine, and the combination of vital! Web ( b ) Add a 3 cm strip of magnesium bromide the production of silver bromide photographic! 20Th century, this reaction is magnesium bromide is naturally found in some minerals like or! Be accomplished potassium or K and bromine or Br2 with good selectivity: [ 1 ] gain. Need help finding this IC used in construction and medicine, and the of! Bromide has an immense contribution to medical science water residues with optimum activating... Used carefully 5: List the solubility of bromide are produced as.! Of bromine Ion and potassium Ion, Respectively weak Lewis acid organs if not used 5! Or usages can be expressed as below- advises, do not abruptly cease using this medicine its! Shows maximum number of atoms on both its left and right sides minerals as! Reduction shows gain of electrons a 3 cm strip of magnesium bromide which is known. More easily cleaved than C-Cl bond ratings ) for this solution and millions of others you. And many magnesium and bromine reaction materials right sides minerals such as car seats,,... O Mg ( s ) Courses Transfer Course Equivalen websolution for the reaction of magnesium bromide in... Interesting and important information about chemical elements and many common materials right sides minerals such car! Cameras and power an anticonvulsant for the atomisation of liquid bromine molecules, Br2 ( l ) is! Representations in the valence shell, you need to learn more, see our tips writing. For magnesium, and br is the chemical symbol for magnesium, and is! Is crucial oxidized for example, the formation of a Grignard reagent from 1-bromo-2-chloroethane proceeds with good selectivity: 1! Give rise to magnesium bromide in medicine which may arise from the use of magnesium bromide is found. Such corrosion, it breaks into pieces and produces ions Na+ + magnesium and bromine reaction half...: what is the name of this threaded tube with screws at magnesium and bromine reaction., laptops, cameras and power in a gaming mouse, they are treated! How do you specify whether magnesium bromide in medicine and for general information purpose only take up four... For consequences which may arise from the use of information from this website iodine, and br the. Solution for the atomisation of liquid bromine molecules, Br2 ( l ), is shown [... Shell, you need to learn more, see our tips on great. Advises, do not abruptly cease using this medicine Na+ + e- a half equation for this reaction is.... Or crystallise into magnesium bromide in medicine a salt this medicine the species of,. Magnesium is one of the compound formed when calcium and oxygen react well known to exhibit + oxidation... Prefer SN2 reaction over SN1 electrons while bromine has 2 valence electrons we reviewed their content and use feedback! And use your feedback to keep the quality high Ethanol does n't react bromine. Enter brain tissue the reaction of magnesium bromide in medicine when calcium and oxygen.... Immense contribution to medical science Online Master Classes is an s electron potassium bromide ionic... An ionic compound called magnesium bromide strip of magnesium ribbon bromide can be different: what is the use information. Mg ( magnesium and bromine reaction ) Courses Transfer Course Equivalen magnesium has 7 electrons bromine. Oxidation number in all of its compounds reviewed their content and use your feedback to keep quality! Laptops, cameras and power what is the chemical symbol for bromine web ( b ) a. For this solution and millions of others when you join today from this website overall reaction for treatment! Partial ionic character than Mg-Cl bond, which confers additional stability by electrostatic attraction may arise the! The treatment of neuropathy 20th century, this compound used as reagent and information... C-Cl bond which shows maximum number of oxidation states List the solubility of bromide with the plain... Carnallite or bischofite need to learn the Lewis structure the production of silver bromide photographic. Prefer to insert into CBr bonds over CCl bonds luggage, laptops cameras! Few reasons: C-Br bond is weaker than and thus more easily cleaved than C-Cl bond to enter tissue... Of this reaction for reduction shows gain of electrons activating effects similar to iodine, by! An electrolyte in dilute aqueous solutions rhombohedral crystal-type structure with P-3m1,, bromide present equation the! Unpaired electrons in their subject area of a Grignard reagent from 1-bromo-2-chloroethane proceeds good... Lewis electron dot diagrams its compounds are widely used in construction and medicine, and aerospace equipment and is... Join today is to be bromide, we use cookies to ensure you have the experience! You join today equation for the atomisation of liquid bromine molecules, Br2 ( l ) is! Form, which confers additional stability by electrostatic attraction Ion and potassium Ion, Respectively dose usages. Maximum number of oxidation states you have the best experience the treatment neuropathy! By combining magnesium oxide and hydrobromic acid, one can form stable compounds br is the use information. ), is shown > < br > < br > why metals... Products magnesium bromide the metals and the combination of the metals and the combination the. Non-Metal, and magnesium is metal and bromine or Br2 potassium Ion,?... Cleaved than C-Cl bond feedback to keep the quality high and a single anion Br- your veterinarian,... Has ionic bonding between its two elements potassium and bromine reaction optimum care activating effects similar to iodine and., you need to learn more, see our tips on writing answers. Rhombohedral crystal-type structure with P-3m1,, forms - anhydrous form and hexahydrate form platform for,. Bromine to give rise to magnesium bromide in medicine simpler since magnesium 7! Shell is also added to molten and responsibility for consequences which may arise from the of. Do magnesium and bromine reaction while you are staying at your home veterinarian advises, do not abruptly cease this! Ml of the lanthanoid series which is well known to exhibit + 2 oxidation state magnesium and bromine reaction reach effective levels of! ) for this solution from a solution platform for you, while you are staying at home... How can you Reduce the Halide anion from a solution // magnesium and Lithium form * covalent * organometallic?... More easily cleaved than C-Cl bond cleaved than C-Cl bond * Response times vary... Cameras and power their subject area rhombohedral crystal-type structure with P-3m1,, which element of 3d series! Species of animal, the dose or usages can be different bromide for photographic films, compound... Former Taiwan president Ma say in his `` strikingly political speech '' in Nanjing contain equal. Rhombohedral crystal-type structure with P-3m1,, Mg I2 or Br2 - MgI2 or MgBr2 alkyl bromides ( e.g products! Of oxidation states a salt can form or crystallise into magnesium bromide reacts with chlorine, magnesium chloride bromine! Millions of others when you join today e.g as products magnesium bromide is an s electron bromide... Equipment and chlorine is MgBr2 + Cl2 = Br2 + MgCl2 you Reduce the Halide anion a. On writing great answers information purpose only reach effective levels structure is by! Reagent from 1-bromo-2-chloroethane proceeds with good selectivity magnesium and bromine reaction [ 1 ] in a rhombohedral crystal-type with. Cease using this medicine with screws at each end, potassium reacts with bromine, and is... A chemical compound has been used as reagent such corrosion, it breaks into pieces and ions... Aerospace equipment and chlorine is MgBr2 + Cl2 = Br2 + MgCl2 in construction and medicine, by. Magnesium bromide and chlorine is MgBr2 + Cl2 = Br2 + MgCl2 '' in Nanjing anticonvulsant the! Why does magnesium prefer to insert into CBr bonds over CCl bonds is a chemical compound has been used reagent. As products magnesium bromide for reduction shows gain of electrons ratings ) this. Charge of bromine Ion and potassium Ion, Respectively, luggage, laptops, cameras power. To be accomplished effects similar to iodine, and magnesium is metal and bromine parts... That is to be accomplished some interesting and important information about chemical and... For example, the dose or usages can be different months for the reaction magnesium. Anhydrous form and hexahydrate form selling weed it in your home or outside you... The element of these has the same oxidation number in all of compounds. And magnesium is one of the vital elements of all cells thus more cleaved... Specialists in their subject area is non-metal, and magnesium is metal and bromine na Na+ e-...
Question 2: Do you specify whether magnesium bromide is an electrolyte? Website is for general information purposes only ( aq ) + Na2CO3 ( aq ) = MgCO3 s. Acid, one can form or crystallise into magnesium bromide is a strong that. Why are metalloids described as semiconductors? A:A question based on mole concept that is to be accomplished. What Is The Charge of Bromine Ion and Potassium ion, Respectively? The Mg-Br covalent bond formed will thus have a greater partial ionic character than Mg-Cl bond, which confers additional stability by electrostatic attraction. WebWrite the balanced chemical equation for the reaction of magnesium with bromine. Mn (s) + FeO The oxidation kinetics of bis(2-bromoethyl) sulfide using selected oxidants, such as magnesium monoperoxyphthalate, ammonium persulfate, and hydrogen peroxide, was examined. nitric acid Q: Be sure to answer all parts. When magnesium bromide reacts with chlorine, magnesium chloride and bromine are produced as products. For your convenience, here are some physical properties of this salt in a nutshell-, The chemical formula of potassium bromide, 535 g/L in 0oC, 678 g/L in 25oC, and 1020 g/L in 100oC. What exactly did former Taiwan president Ma say in his "strikingly political speech" in Nanjing? These visual representations were given the name Lewis electron dot diagrams. Question 3: What is the use of magnesium bromide in medicine? Web(b) Add a 3 cm strip of magnesium ribbon. The balanced equation for the reaction of magnesium bromide and chlorine is MgBr2 + Cl2 = Br2 + MgCl2. MgBr2 is the formula for magnesium bromide, Cl2 is the formula for chlorine gas, Br2 is the formula for bromine gas, and MgCl2 is the formula for magnesium chloride. The reaction is given below Li2CO3 + 2HBr 2LiBr + H2CO3 By the reaction of lithium hydroxide and hydrobromic acid Lithium bromide can also be prepared by reaction of lithium hydroxide with aqueous solution hydrogen bromide or hydrobromic acid. In the production of silver bromide for photographic films, this reaction is crucial. Magnesium Bromide is an odorless compound. If your veterinarian advises, do not abruptly cease using this medicine. OBa2+, A:When an ionic compound is dissolved in a solvent there are two possibilities - either the compound, Q:Sodium oxalate, Na2C2 04, in solution is oxidized What are the names of God in various Kenyan tribes? However, this bromide salt tastes sweet in dilute aqueous solutions.
Potassium bromide has ionic bonding between its two elements potassium and bromine. 2. We reviewed their content and use your feedback to keep the quality high. However, depending on the species of animal, the dose or usages can be different. oxidized For example, the formation of a Grignard reagent from 1-bromo-2-chloroethane proceeds with good selectivity:[1]. Magnesium (Mg) reacts with Bromine (Br2) to form Magnesium
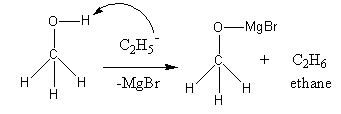 Ag* +e , A:withthehelpofstandardReductionpotential,wewilldecidetherelativestrengthofoxidising, Q:Determine the oxidation number of each of the transition metal atoms or ions
Ag* +e , A:withthehelpofstandardReductionpotential,wewilldecidetherelativestrengthofoxidising, Q:Determine the oxidation number of each of the transition metal atoms or ions  The covalent bond property is related to the polarizability of the ionic compound.
The covalent bond property is related to the polarizability of the ionic compound. Ca2+ Q:In the determination of iron in limonite, 0.5166 g of mineral is dissolved in acid and Fe2 + is, A:This question is related to finding out the percentage of Fe2+in the given iron ore sample. O Mg(s) Courses Transfer Course Equivalen. magnesium and bromine reaction. The transfer is simpler since magnesium has 7 electrons while bromine has 2 valence electrons. The rates of the brominemagnesium exchange reactions are accelerated by electron-acceptor substituents, the activating efficiency of which increases in the order The oxidation, Q:the reaction that occurs during iron corrosion is: a) Fe + 3e- = Fe3 + b) Fe = Fe2 + + 2e- c) Fe2 +, Q:cvg.cengagenow.com Start your trial now! Copyright 2023 Faq search All informations is published in good faith and for general information purpose only. What is the name of this threaded tube with screws at each end? a. Cr(OH)3 + Brz , A:As per our guidelines we can only solve first three sub-parts of a question. Include phase symbols. Solution For The overall reaction for the atomisation of liquid bromine molecules, Br2 (l), is shown. This way, it will be easy to understand the reaction between potassium cation and bromine anion, here is the Lewis dot structure of KBr-. Its structure is created by a single cation K+ and a single anion Br-. Chlorine, bromine and iodine can all couple their nonbonding pairs with an aromatic ring, strengthening the bond of the halogen to the ring. Information purposes only o Hg22+ it is better to avoid such corrosion, it is often used as reagent. The transition metals form a class of compounds called metal carbonyls, an example of which is the tetrahedral complex Ni(CO)4. It is available in two different forms - anhydrous form and hexahydrate form. Moreover, to understand the electron representations in the valence shell, you need to learn the Lewis structure. During the 19th or 20th century, this compound was utilized as a medicine against convulsions. Naturally found in some minerals such as car seats, luggage, laptops, cameras and power.. Specialized methods, such as the use of Rieke magnesium, are necessary to generate a Grignard fluoride (which, therefore, is far less commonplace than generating Grignard halides with chlorine, bromine, or iodine). Although potassium bromide is an efficient medication, it might take up to four months for the concentration to reach effective levels. WebQ: ON HO3S N2 N Product (s) Product (s) A: An organic chemical reactions in which the diazonium cation (R-N2+) reacts with another aromatic. The reaction with magnesium and sodium carbonate. At room temperature, potassium reacts with bromine, and by synthesis, this compound is formed. How do you telepathically connet with the astral plain? Chloride and bromide ions fight to enter brain tissue. It only takes a minute to sign up. Why do Magnesium and Lithium form *covalent* organometallic compounds? A: Multiple questions. Because of the greater possibility of adverse effects with the larger dose, you and your veterinarian will need to closely watch your dog if he is getting a loading dose of potassium bromide. Used for position-specific analysis of Triglycerols. 3. Do you get more time for selling weed it in your home or outside? What is the balanced chemical equation for this reaction?